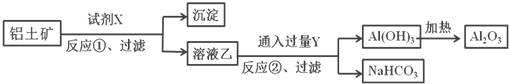
��Ŀ����
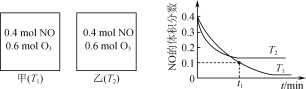
����Ŀ��N2H4��N2O4���������������ƽ������ƽ���������Ӧ��2N2H4��l����N2O4��l��=3N2��g����4H2O��g���������й�˵����ȷ���ǣ�������
A.��Ӧÿ����0.3 mol N2��ת�Ƶ��ӵ���ĿΪ1.6��6.02��1023
B.����N2O4���ܱ������д��ڣ�N2O4��g��![]() 2NO2��g������c��N2O4����c��NO2����1��2ʱ���ÿ��淴Ӧ������
2NO2��g������c��N2O4����c��NO2����1��2ʱ���ÿ��淴Ӧ������
C.����N2H4������ȼ�ϵ�ع���ʱ�������缫��ӦʽΪO2��2H2O��4e��=4OH��
D.N2H4��ˮ��Һ�д��ڣ�N2H4��H2O![]() N2H
N2H![]() ��OH������ϡ��Һ�м�ˮϡ�ͣ�
��OH������ϡ��Һ�м�ˮϡ�ͣ� ![]() ��ֵ���
��ֵ���
���𰸡�C
��������
A��2N2H4��l����N2O4��l��=3N2��g����4H2O��g����ÿ����3molN2ת�Ƶ�������8mol����Ӧÿ����0.3 mol N2��ת�Ƶ��ӵ���ĿΪ0.8��6.02��1023����A����
B������N2O4���ܱ������д��ڣ�N2O4��g��![]() 2NO2��g������c��N2O4����c��NO2����1��2ʱ����ȷ�����淴Ӧ�����Ƿ���ȣ���֤����Ӧ�Ƿ�ﵽƽ��״̬����B����
2NO2��g������c��N2O4����c��NO2����1��2ʱ����ȷ�����淴Ӧ�����Ƿ���ȣ���֤����Ӧ�Ƿ�ﵽƽ��״̬����B����
C������N2H4������ȼ�ϵ�ع���ʱ��������������O2�õ��ӣ�������ԭ��Ӧ�������缫��ӦʽΪO2��2H2O��4e��=4OH������C��ȷ��
D��N2H4��ˮ��Һ�д��ڣ�N2H4��H2O![]() N2H
N2H![]() ��OH������ϡ��Һ�м�ˮϡ�ͣ�c(N2H4)��С��c(OH-)��С��c(H+)����
��OH������ϡ��Һ�м�ˮϡ�ͣ�c(N2H4)��С��c(OH-)��С��c(H+)����![]() ��ֵ��С����D����
��ֵ��С����D����
��ѡC��

����Ŀ�����ĺϳ�ԭ��Ϊ:N2��g����3H2��g��2NH3��g��H=��92.4kJ��mol-1��500����20MPaʱ����N2��H2����һ���ݻ�Ϊ2L���ܱ������з�����Ӧ����Ӧ�����и����ʵ����ʵ����仯��ͼ���ش���������:
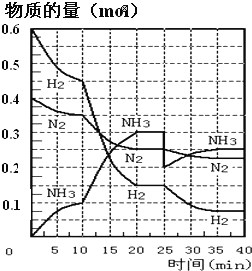
��1��10min����NH3��ʾ��ƽ����Ӧ����Ϊ_________
��2����10��20min�ڣ�NH3Ũ�ȱ仯��ԭ�������_____________
A.���˴���
B.���������
C.�����¶�
D.����NH3���ʵ���
��3����1��ƽ��:ƽ�ⳣ��K1=__________�������ݵı���ʽ������2��ƽ��ʱNH3���������Ϊ_____________��
��4�������������˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fc2O3��TiO2��������ˮ�������з�Ӧ:N2��g����3H2O��l��2NH3+1.5O2��g��H=akJ��mol-1����һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ���������±�:
T/K | 303 | 313 | 323 |
NH3������/(10-6mol) | 4.8 | 5.9 | 6.0 |
�ٴ˺ϳɷ�Ӧ��a______0����S_______0����������������������һ����
����֪N2��g����3H2��g��2NH3��g��H=��92.4kJ��mol-1��2 H2��g���� O2��g��=2H2O��l��H=-571.kJ��mol-1 �����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ_____________________________
����Ŀ��ʵ�����Թ�ҵ����Ϊԭ����ȡCoSO4��Һ��ZnSO4��7H2O���壬��ʵ���������£�
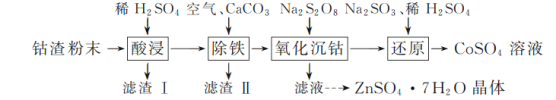
��֪���١������������Һ����Ҫ��CoSO4��ZnSO4����������FeSO4��NiSO4����������ԣ�Ni����Fe��Cu֮�䡣���±�����ؽ������������������������pH����ʼ������pH����������Ũ��1 mol��L��1���㣬pH��11ʱZn��OH��2������NaOH��Һ����[Zn��OH��4]2����:
�������� | ��ʼ������pH | ��ȫ������pH |
Co2�� | 7.6 | 9.4 |
Zn2�� | 5.9 | 8.9 |
��1���������ʱ����ϡ����˹���̫���ԭ����________��
��2����������ʱ������Һ�г������������������________��
��3���������轫����������������ϴ��Һ������������������Һ�ϲ���Ŀ����________��
��4��д������ԭ��������Na2SO3��ϡH2SO4��Co��OH��3��Ӧ�����ӷ���ʽ��________��
��5��ʵ������CoSO4��Һ�������Ʊ�CoCO3���Ʊ�ʱCoSO4������Һ��Na2CO3������Һ�Ļ�Ϸ�ʽΪ________��
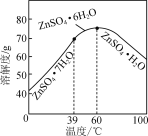
��6��������ͼ����п������ܽ�����ߣ���ƴ������������������Һ����ZnSO4��Na2SO4��NiSO4�ȣ��л�ȡZnSO4��7H2O��ʵ�鷽����ȡ������Һ��____________________________�����ˣ���������ˮϴ�ӣ����¸����ZnSO4��7H2O���塣��ʵ������ʹ�õ��Լ��У�Zn�ۡ�1.0 mol��L��1 NaOH��1.0 mol��L��1 H2SO4��
����Ŀ���ں��������£���Ӧ��2SO2 (g) + O2 (g) ![]() 2SO3(g) ��H =��QkJ��mol��1�������������·ֱ���������ͷ�Ӧ�ų������� ( Q>0 )���±����У�
2SO3(g) ��H =��QkJ��mol��1�������������·ֱ���������ͷ�Ӧ�ų������� ( Q>0 )���±����У�
���� | SO2 (mol) | O2(mol) | N2(mol) | Q(kJ��mol��1) |
�� | 2 | 1 | 0 | Q1 |
�� | 1 | 0.5 | 0 | Q2 |
�� | 1 | 0.5 | 1 | Q3 |
�����������ݣ�����������ȷ���ǣ� ��
A. �����������·�Ӧ����lmol SO3�������Q/2 kJ B. 2Q3 <2Q2=Q1<Q
C. Ql =2Q2= 2Q3 = Q D. 2Q2 = 2Q3 < Q1 < Q