��Ŀ����
���и���Һ�У����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A����0.1mol?L-1 CH3COONa��Һ�У�c��OH-��=c��CH3COOH��+c��H+�� |
| B��0.1mol?L-1��KAl��SO4��2��Һ�У�c��SO42-����c��Al3+����c��OH-����c��H+�� |
| C��pH��ȵ�NaOH��CH3COONa��NaHCO3������Һ�����У�c��NaOH����c��CH3COONa����c��NaHCO3�� |
| D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ������Һ�����ԣ�����c��Na+����c��HX����c��X-����c��H+����c��OH-�� |
���㣺����Ũ�ȴ�С�ıȽ�
ר�⣺�����ˮ��ר��
������A���κ���Һ�ж����������غ㣬���������غ��жϣ�
B������������ǿ�������Σ�����Һ�����ԣ�
C��pH��ȵ�NaOH��CH3COONa��NaHCO3������Һ��NaOH��ǿ���Ũ����С��������Һ���������ˮ��̶�Խ������Ũ��ԽС��
D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ������Һ�����ԣ�˵��HX�ĵ���̶ȴ���X-��ˮ��̶ȣ�
B������������ǿ�������Σ�����Һ�����ԣ�
C��pH��ȵ�NaOH��CH3COONa��NaHCO3������Һ��NaOH��ǿ���Ũ����С��������Һ���������ˮ��̶�Խ������Ũ��ԽС��
D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ������Һ�����ԣ�˵��HX�ĵ���̶ȴ���X-��ˮ��̶ȣ�
���
�⣺A���κ���Һ�ж����������غ㣬���������غ��c��OH-��=c��CH3COOH��+c��H+������A��ȷ��
B������������ǿ�������Σ�������ˮ�������Һ�����ԣ�����c��H+����c��OH-������B����
C��pH��ȵ�NaOH��CH3COONa��NaHCO3������Һ��NaOH��ǿ���Ũ����С��������Һ���������ˮ��̶�Խ������Ũ��ԽС���������ˮ��̶�HCO3-��CH3COO-������������ҺŨ�ȴ�С˳����c��NaOH����c��NaHCO3����c��CH3COONa������C����
D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ������Һ�����ԣ�˵��HX�ĵ���̶ȴ���X-��ˮ��̶ȣ�����Һ�д���c��Na+����c��X-����c��HX������D����
��ѡA��
B������������ǿ�������Σ�������ˮ�������Һ�����ԣ�����c��H+����c��OH-������B����
C��pH��ȵ�NaOH��CH3COONa��NaHCO3������Һ��NaOH��ǿ���Ũ����С��������Һ���������ˮ��̶�Խ������Ũ��ԽС���������ˮ��̶�HCO3-��CH3COO-������������ҺŨ�ȴ�С˳����c��NaOH����c��NaHCO3����c��CH3COONa������C����
D��������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����Һ������Һ�����ԣ�˵��HX�ĵ���̶ȴ���X-��ˮ��̶ȣ�����Һ�д���c��Na+����c��X-����c��HX������D����
��ѡA��
���������⿼��������Ũ�ȴ�С�Ƚϣ�������Һ���ʵ�����ȷ����Һ����ԡ������������ˮ��̶�ȷ��������Һ����ǿ�����ٽ���غ�˼����������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
�����Ŀ
ij�л����м���ṹ��ͼ��ʾ�������ܷ����ķ�Ӧ�����ǣ�������
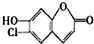

| A���ӳɷ�Ӧ | B��ȡ����Ӧ |
| C��ˮ�ⷴӦ | D����ȥ��Ӧ |
�鿴���о�����Ʒ����Ʒ��ǩ�����оƾ�������ߵ��ǣ�������
| A��ơ�� | B���� | C�����Ѿ� | D���ƾ� |
��֪���з�Ӧ��
Co2O3+6HCl��Ũ���T2CoCl2+Cl2��+3H2O
2FeCl3+2KI�T2FeCl2+I2+2KCl Cl2+2FeCl2�T2FeCl3
������������������ǿ������˳���ǣ�������
Co2O3+6HCl��Ũ���T2CoCl2+Cl2��+3H2O
2FeCl3+2KI�T2FeCl2+I2+2KCl Cl2+2FeCl2�T2FeCl3
������������������ǿ������˳���ǣ�������
| A��I2��FeCl3��Cl2��Co2O3 |
| B��Co2O3��Cl2��FeCl3��I2 |
| C��Cl2��Co2O3��I2��FeCl3 |
| D��Cl2��I2��Co2O3��FeCl3 |
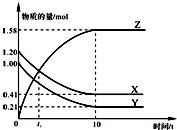 һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ������������ȷ���ǣ�������
һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯��������ͼ��ʾ������������ȷ���ǣ�������| A����Ӧ��ʼ��10 s����Z��ʾ�ķ�Ӧ����Ϊ0.158 mol/��L?s�� |
| B����Ӧ��ʼ��10 sʱ��Y��ת����Ϊ79.0% |
| C��t1ʱ��Z��X��Ũ����ȣ��ﵽ�˻�ѧƽ��״̬ |
| D����Ӧ�Ļ�ѧ����ʽΪ��X��g��+Y��g��?Z��g�� |
��NA���������ӵ�������ֵ�������й�������ȷ���ǣ�������
| A��1mol CH4��g����2mol O2��g���������ܺ�С��1mol CO2��g����2mol H2O��g���������ܺ� |
| B����״���£�44.8L NO��22.4L O2��Ϻ������з�������С��2NA |
| C��1mol Fe�������г��ȼ��ʧȥ3NA������ |
| D���ڱ�״���£�NA��CHCl3������ռ�����ԼΪ22.4L |
�������ӷ���ʽ��������ʵ�������ȷ���ǣ�������
| A��Ca��HCO3��2��Һ�м�������NaOH��Һ��Ca2++2HCO3-+2OH-�TCaCO3��+CO32-+H2O | ||||
| B����������������ϡ���3Fe2++4H++NO3-�T3Fe3++NO��+3H2O | ||||
| C������0.4mol FeBr2����Һ��ͨ��0.3mol Cl2��ַ�Ӧ��4Fe2++2Br-+3Cl2�T4Fe3++6Cl-+Br2 | ||||
D����ͭΪ�缫��ⱥ��ʳ��ˮ��2Cl-+2H2O
|
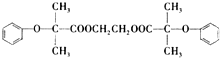 ����������·�ߺϳɣ�
����������·�ߺϳɣ�
