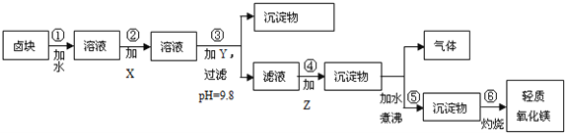
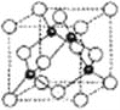
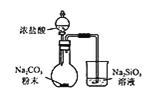
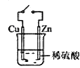
��Ŀ����
����Ŀ��(1)�״�![]() ����Ҫ���ܼ������ȼ�ϣ���ҵ����CO��H2��һ���������Ʊ�
����Ҫ���ܼ������ȼ�ϣ���ҵ����CO��H2��һ���������Ʊ�![]() �ķ�Ӧ��
�ķ�Ӧ��![]() �������Ϊ1L�ĺ����ܱ������У���2molCO��
�������Ϊ1L�ĺ����ܱ������У���2molCO��![]() ��һ�������·���������Ӧ�����
��һ�������·���������Ӧ�����![]() ��
��![]() ��Ũ����ʱ��仯��ͼ��ʾ��
��Ũ����ʱ��仯��ͼ��ʾ��
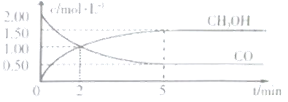
�ٴӷ�Ӧ��ʼ��5min����һ����̼��ʾ��ƽ����Ӧ��������CO��=______��
������˵����ȷ����______��
A.�ﵽƽ��ʱ��![]() ��ת����Ϊ
��ת����Ϊ![]()
B.5min�������л�������ƽ����Է����������ٸı�
C.�ﵽƽ����ٳ��백������Ӧ���ʼ�С
D.2minǰ��������>�����棩��2min����������<�����棩
(2)һ���¶��£���![]() ��
��![]() �������1��2�����ܱ������з�����Ӧ��
�������1��2�����ܱ������з�����Ӧ��
![]() ���ﵽƽ��ʱ
���ﵽƽ��ʱ![]() ���������Ϊ
���������Ϊ![]() ���÷�Ӧ��ƽ�ⳣ��
���÷�Ӧ��ƽ�ⳣ��![]() ______��
______��
(3)̼��ˮ������Ӧ��ȡ![]() ����ط�Ӧ���£�
����ط�Ӧ���£�
��![]()
��![]()
��![]()
�ټ��㷴Ӧ![]() ��
��![]() ______
______![]()
�ڶ��ڿ��淴Ӧ![]() ����ȡ���´�ʩ�������
����ȡ���´�ʩ�������![]() ���ʵ���______��
���ʵ���______��
A.������ϵ���¶�
B.ѹ�����������
C.����CaO����
D.ѡ���ʵ��Ĵ���
(4)�Լ״�Ϊȼ�ϣ�![]() Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ��
Ϊ��������KOH��ҺΪ�������Һ�����Ƴ�ȼ�ϵ��![]() �缫����Ϊ���Ե缫
�缫����Ϊ���Ե缫![]() ����KOH��Һ������д����ȼ�ϵ�ظ����ĵ缫��Ӧʽ��______��
����KOH��Һ������д����ȼ�ϵ�ظ����ĵ缫��Ӧʽ��______��
(5)����20mL![]() ������
������![]() ��Һ����μ���һ��Ũ�ȵ��ռ���Һ����û����Һ���¶ȱ仯��ͼ��ʾ�������й�˵����ȷ����______
��Һ����μ���һ��Ũ�ȵ��ռ���Һ����û����Һ���¶ȱ仯��ͼ��ʾ�������й�˵����ȷ����______
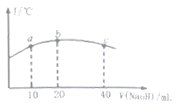
�ٸ��ռ���Һ��Ũ��Ϊ![]()
�ڸ��ռ���Һ��Ũ��Ϊ![]()
��![]() �ĵ���ƽ�ⳣ����b��
�ĵ���ƽ�ⳣ����b��![]() ��
��
�ܴ�b�㵽c�㣬�����Һ��һֱ���ڣ�c(Na+)>c(NO2-)>c(OH-)>c(H+)
���𰸡�![]() B
B ![]()
![]() A
A ![]() �ڢ�
�ڢ�
��������
��1���ٸ���ͼ�������ݺͻ�ѧ��Ӧ���ʵĹ�ʽ���㣻
�ڽ��ͼ����й����ݹ�ϵ���м��㣬���ݻ�ѧƽ��״̬�ı�־�����жϣ�
��2����������ʽ���м��㣻
��3���ٸ��ݸ�˹���ɽ��м��㣻
�ڸ���Ӱ�컯ѧƽ���ƶ������ط����жϣ�
��4��CH3OH�ڸ�������ʧ���ӵ�������Ӧ���ݴ���д�缫��Ӧʽ��
��5��b���¶���ߣ�˵����ʱNaOH��NaNO2ǡ����ȫ��Ӧ���ݴ˷����жϡ�
��1���ٸ���ͼ������CO��=![]() mol/(L��min)=0.3 mol/(L��min)��
mol/(L��min)=0.3 mol/(L��min)��
��A.����ƽ��ʱ���������������ʵ���Ũ��Ϊ2��(2��0.5)mol��L��1=3mol��L��1��������ת����Ϊ![]() ��100%=75%��ѡ��A����
��100%=75%��ѡ��A����
B.������������ʼ�ղ��䣬���÷�Ӧ���������ʵ������ٵķ�Ӧ����˻�������ƽ����Է�����������ʱ��˵���ﵽƽ�⣬��5minʱ����Ӧ�ﵽƽ�⣬ѡ��B��ȷ��
C.����״̬�£�����NH3�������Ũ�Ȳ��䣬��ѧ��Ӧ���ʲ��䣬ѡ��C����
D.5minʱ��Ӧ�ﵽƽ�⣬5minǰ��Ϊ��( ��)>��(��)��ѡ��D����
��ѡB��
��2����Ϊ�÷�Ӧ��Ӧǰ��������������䣬����NO2�����ʵ���Ũ��Ϊ1mol/L��SO2�����ʵ���Ϊ2mol/L��
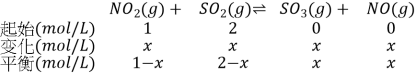
��������ó�![]() ��100%=25%�����x=0.75�����ݻ�ѧƽ�ⳣ���Ķ��壬
��100%=25%�����x=0.75�����ݻ�ѧƽ�ⳣ���Ķ��壬![]() �������K=
�������K=![]() =1.8��
=1.8��
��3���ٸ��ݸ�˹���ɣ���I+II��III�ó���H=(131��43��178.3)kJ��mol��1=��90.3kJ��mol��1��
��A.����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ��������Ӧ������У������IJ������ӣ�ѡ��A�ɲ�ȡ��
B.��Ӧǰ������ϵ��֮����ȣ�ѹ�������������ƽ�ⲻ�ƶ�����H2�IJ��ʲ��䣬ѡ��B���ɲ�ȡ��
C.CaO�ǹ��壬Ũ����Ϊ������������CaO����ƽ�ⲻ�ƶ���H2�IJ��ʲ��䣬ѡ��C���ɲ�ȡ��
D.������ƽ����ƶ���Ӱ�죬H2�IJ��ʲ��䣬ѡ��D���ɲ�ȡ��
��ѡA��
��4���Լ״�Ϊȼ�ϣ�![]() Ϊ��������KOH��ҺΪ�������Һ��ͨ�״�һ��Ϊ�����������缫��ӦʽΪ
Ϊ��������KOH��ҺΪ�������Һ��ͨ�״�һ��Ϊ�����������缫��ӦʽΪ![]() ��
��
��5���٢ڵ�����20mL����������Һʱ���¶ȴﵽ��ߣ�����ǡ����ȫ��Ӧ����NaOH��Ũ��Ϊ0.01mol��L-1���ٴ�����ȷ���۵���ƽ�ⳣ�����¶ȵ�Ӱ�죬������ʵĵ��������ȹ��̣������¶ȴٽ����룬b���¶ȱ�a��ߣ����b��HNO2�ĵ���ƽ�ⳣ����a�����ȷ���ܴ�b�㵽c�㣬��c����Һ������ΪNaNO2��NaOH�����������ʵ�����ȣ���ʱ������Ũ�ȴ�С˳���ǣ�c(Na��)>c(OH��)>c(NO2��)>c(H��)���ܴ���
��ѡ�ڢۡ�

 ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�����Ŀ�����и���ʵ������ó��Ľ�����ȷ����![]()
ѡ�� | ʵ����� | ���� | ���� |
A | �� | �õ�������Һ | X��һ������ |
B | Ũ�Ⱦ�Ϊ | ������ɫ���� |
|
C |
| ��ֽ��Ϊ��ɫ |
|
D |
| �л���ʳ�ɫ | �����ԣ� |
A.AB.BC.CD.D