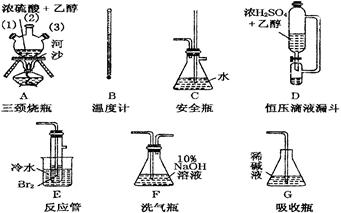
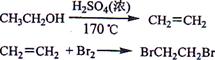
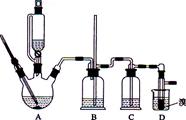
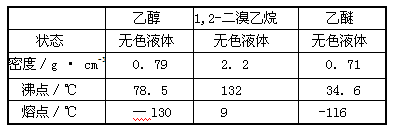
��Ŀ����
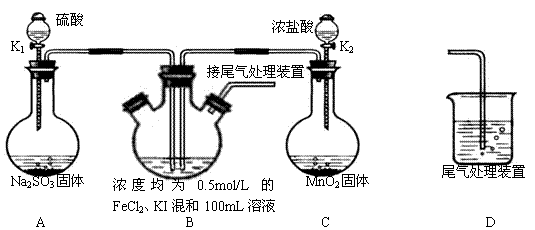
ijʵ��С��������ϵ�֪��
5C2O42����2MnO4����16H��====10CO2����2Mn2����8H2O
�����ø÷�Ӧ�ⶨij�����ƣ�Na2C2O4����Ʒ�в����Ƶ�������������С�����1.34 g��������Ʒ����ϡ�����У�Ȼ����0.200 mol��L-1�����Ը��������Һ���еζ������е����ʲ���������غ�ϡ���ᷴӦ����
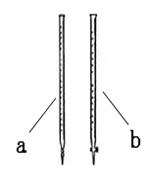
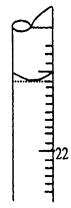
��1���ζ�ʱ��______����a��b����
��2���ζ���ʢװKMnO4����Һ���ζ�ǰ______����ǡ����μ�ָʾ�����ζ��յ��������____________________��
��3���ﵽ�յ�ʱ������15.00 mL�ĸ��������Һ����Ʒ�в����Ƶ���������Ϊ______________��
5C2O42����2MnO4����16H��====10CO2����2Mn2����8H2O
�����ø÷�Ӧ�ⶨij�����ƣ�Na2C2O4����Ʒ�в����Ƶ�������������С�����1.34 g��������Ʒ����ϡ�����У�Ȼ����0.200 mol��L-1�����Ը��������Һ���еζ������е����ʲ���������غ�ϡ���ᷴӦ����

��1���ζ�ʱ��______����a��b����
��2���ζ���ʢװKMnO4����Һ���ζ�ǰ______����ǡ����μ�ָʾ�����ζ��յ��������____________________��
��3���ﵽ�յ�ʱ������15.00 mL�ĸ��������Һ����Ʒ�в����Ƶ���������Ϊ______________��
����5�֣�
��1��b ��1�֣�
��2���� ��1�֣�����Һ��ɫ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٸı䣨1�֣�
��3��75% ��2�֣�
��1��b ��1�֣�
��2���� ��1�֣�����Һ��ɫ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٸı䣨1�֣�
��3��75% ��2�֣�
�����������1��������ؾ���ǿ�����ԣ���ʴ��Ƥ�ܣ�Ӧ����ʽ�ζ��ܣ��ʴ�Ϊ��b��
��2�����������ҺΪ�Ϻ�ɫ�����ﵽ�ζ��յ�ʱ���ٵ�����������Һʱ���Ϻ�ɫ������ȥ���ʴ�Ϊ����KMnO4��Һ���Ϻ�ɫ����ζ��յ���ɫ������ȥ����3�������ƣ�Na2C2O4������ϡ�����У�Ȼ�������Ը��������Һ���еζ������ӷ���ʽΪ��2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O�� n��KMnO4��=0.015L��0.200mol?L-1=3��10-3mol�����ݷ���ʽ�ɵã�
2MnO4-+5C2O42-+16H+=2Mn2++10CO2��+8H2O��
2 5
3��10-3mol 7.5��10-3mol
��Ʒ�в����Ƶ�����Ϊm=7.5��10-3mol��134g/mol=7.5��134��10-3g��
��Ʒ�в����Ƶ���������Ϊ(7.5��134��10-3g)/1.34g��100%=75%��
�ʴ�Ϊ��75%��
������������Ҫ���ʵ�鿼������������Ի�ѧ��Ӧ���ʵ�Ӱ�죬���������ļ��㣬ע�����ʵ���ԭ����Ҫ��߱�һ�������۷��������ͼ����������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ