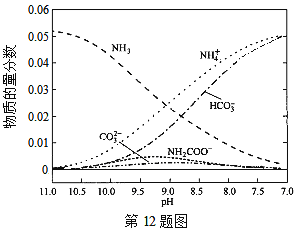
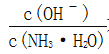��Ŀ����
����Ŀ�������£���0.10 mol��L-1������ε���20.00 mL 0.10 mol��L-1��ˮ�У���Һ��pH��pOH�������������仯������ͼ��ʾ����֪��pOH= -lg c(OH-)������˵����ȷ����
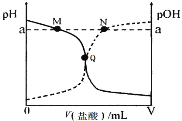
A.M����ʾ��Һc(![]() )+c(NH3��H2O)= c(Cl-)
)+c(NH3��H2O)= c(Cl-)
B.N����ʾ��Һ�У�c(![]() )>c(Cl-)
)>c(Cl-)
C.Q����ʾ���������������ڰ�ˮ�����
D.M���N����ʾ��Һ��ˮ�����ӻ���ͬ
���𰸡�D
��������
��������ĵ�����Һ��pH��С��pOH����������ʵ�ߴ���pH�仯���ߣ����ߴ���pOH�仯���ߣ�Q��pH=pOH����c(OH-)=c(H+)����ʱ��Һ�����ԣ�Q�������ҺΪ���ԣ�Q���Ҳ���ҺΪ���ԡ�
A��M����Һ�ʼ��ԣ�ӦΪNH4Cl��NH3H2O�����Һ����Һ��c(![]() )+c(NH3H2O)>c(Cl-)����A����
)+c(NH3H2O)>c(Cl-)����A����
B��N����Һ�����ԣ���Һ��c(H+)>c(OH-)����ϵ���غ�c(![]() )+c(H+)=c(Cl-)+c(OH-)����֪c(
)+c(H+)=c(Cl-)+c(OH-)����֪c(![]() )<c(Cl-)����B����
)<c(Cl-)����B����
C�������һˮ�ϰ�ǡ����ȫ��Ӧʱ�õ�NH4Cl��Һ�����ԣ�Q����Һ�����ԣ���ˮ�Թ�����������������С�ڰ�ˮ���������C����
D��һ���¶���ˮ�����ӻ�Ϊ�������¶Ȳ���ˮ�����ӻ��������䣬��D��ȷ��
�ʴ�ΪD��

����Ŀ���״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϡ���֪�Ʊ��״����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶�/�� | |
500 | 800 | ||
��2H2(g)��CO(g) | K1 | 2.5 | 0.15 |
��H2(g)��CO2(g) | K2 | 1.0 | 2.50 |
��3H2(g)��CO2(g) | K3 | ||
��1���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��______(��K1��K2��ʾ)��
��2����Ӧ�۵Ħ�H____0(���������)��
��3��500��ʱ��÷�Ӧ����ijʱ��H2(g)��CO2(g)��CH3OH(g)��H2O(g)��Ũ����ȣ��Ҿ�Ϊ0.1mol��L��1�����ʱ����____����(�>������������<��)
��4��ij�¶�����2L�����ܱ������м���CH3OH������Ӧ2CH3OH��g��![]() CH3OCH3��g��+H2O��g��������й��������£�
CH3OCH3��g��+H2O��g��������й��������£�
��Ӧʱ��/min | 0 | 1 | 2 | 3 | 4 |
n��CH3OH��/mol | 1.02 | 0.42 | 0.22 | 0.02 | 0.02 |
�ٷ�Ӧ��2min����CH3OCH3��ʾ�Ļ�ѧ��Ӧ����Ϊ____��
�ڸ��¶��µķ�Ӧ��ƽ�ⳣ��Ϊ____��