��Ŀ����
10���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���Ҫ������Ӧ�Ļ�ѧ������ջش�| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� |
 ��
����2���ۢݢ�Ԫ���γɵļ����ӣ����Ӱ뾶��С�����˳��Al3+��F-��Cl-�������ӷ��ţ�
��3���ܢ�Ԫ���γɵĻ�����ĵ���ʽΪ
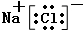 ����Ԫ���γɵĵ�����ṹʽΪN��N��
����Ԫ���γɵĵ�����ṹʽΪN��N����4���û�ѧʽ�������������Ƚϣ��ڢ���Ԫ����̬�⻯����ȶ���NH3��HF���ޢ���Ԫ������������Ӧˮ��������H2SiO3��HClO4��
��5���ܢ���Ԫ������������Ӧˮ�������Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
���� ��Ԫ�������ڱ���λ�ã���֪��ΪBe����ΪN����ΪF����ΪNa����ΪAl����ΪSi����ΪCl����ΪAr��
��1��ͬ����������ҷǽ�������ǿ��ͬ�������϶��·ǽ����Լ�����ϡ������Ar��ѧ��������ã�
��2�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵ��Ӳ�Խ�࣬���Ӱ뾶Խ��
��3���ܢ�Ԫ���γɵĻ�����ΪNaCl�����������������ӹ��ɣ���Ԫ���γɵĵ���ΪN2��������Nԭ��֮���γ�3�Թ��õ��Ӷԣ�
��4��Ԫ�طǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�������������Ӧˮ��������Խǿ��
��5���ܡ�����Ԫ������������Ӧˮ����ֱ�ΪNaOH��Al��OH��3�����߷�Ӧ����ƫ��������ˮ��
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪBe����ΪN����ΪF����ΪNa����ΪAl����ΪSi����ΪCl����ΪAr��
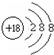
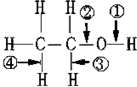
��1����ΪBe������Ϊ�룬ͬ����������ҷǽ�������ǿ��ͬ�������϶��·ǽ����Լ�������FԪ�طǽ�������ǿ��ϡ������Ar��ѧ��������ã���ԭ�ӽṹʾ��ͼΪ�� ��
��
�ʴ�Ϊ���룻F�� ��
��
��2�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����ӵ��Ӳ�Խ�࣬���Ӱ뾶Խ�����Ӱ뾶��Al3+��F-��Cl-��
�ʴ�Ϊ��Al3+��F-��Cl-��
��3���ܢ�Ԫ���γɵĻ�����ΪNaCl�����������������ӹ��ɣ�����ʽΪ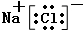 ����Ԫ���γɵĵ���ΪN2��������Nԭ��֮���γ�3�Թ��õ��Ӷԣ��ṹʽΪ��N��N��
����Ԫ���γɵĵ���ΪN2��������Nԭ��֮���γ�3�Թ��õ��Ӷԣ��ṹʽΪ��N��N��
�ʴ�Ϊ��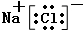 ��N��N��
��N��N��
��4���ǽ�����N��F�����⻯���ȶ��ԣ�NH3��HF���ǽ�����Si��Cl������������Ӧˮ��������H2SiO3��HClO4��
�ʴ�Ϊ��NH3��HF��H2SiO3��HClO4��
��5���ܡ�����Ԫ������������Ӧˮ����ֱ�ΪNaOH��Al��OH��3�����߷�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��ѶȲ���ע���Ԫ�������ɵ��������գ�ע������ԡ��ǽ�����ǿ���Ƚ�ʵ����ʵ��

�ٷ�Ӧ������ѹǿ����ʱ��仯���仯
��A�����B����������������
�ۻ�������ƽ��Ħ����������ʱ��仯���仯
�ܷ�Ӧ���������ܶȲ���ʱ��仯���仯��
| A�� | �ڢ� | B�� | �ۢ� | C�� | �٢� | D�� | �٢� |
| A�� | ���ô�����������ʱ�����ƻ��ļ��Ǣڢ� | |
| B�� | ���ô���Na��Ӧʱ�����ƻ��ļ��Ǣ� | |
| C�� | ���ô������ᷢ��������Ӧʱ�����ƻ��ļ��Ǣ� | |
| D�� | ���ô�������ȥ��Ӧʱ�����ƻ��ļ��Ǣٺ͢� |
| A�� | Na2O2��ˮ��Ӧ��O2��ÿ����0.25molO2ת�Ƶ�����Ϊ1NA | |
| B�� | �����ʵ�����N2��CO����ԭ������Ϊ2NA | |
| C�� | �ڱ�״���£�11.2L HCHO�����ķ�����ԼΪ0.5NA | |
| D�� | 1molSO2��������O2��Ӧ��ת�Ƶĵ�����Ϊ2NA |
| A�� | CO | B�� | H2 | C�� | CO2 | D�� | Cl2 |
| A�� | ϡ��Ũ����ʱ��Ӧ������ˮ�ز���������ע��Ũ������ | |
| B�� | ��SO2��Cl2�������ʵ�������ͨ��Ʒ����Һ�У�Ʒ��ܿ���ɫ | |
| C�� | NO2��H2O��Ӧ�����У��������ĵ�ԭ���뱻��ԭ�ĵ�ԭ�Ӹ�����Ϊ2��1 | |
| D�� | ����ij��Һ�Ƿ���SO42-ʱ��Ӧȡ��������Һ�����μ���BaCl2��Һ��ϡ���� |
| A�� | ���³�ѹ�£�32g����������ԭ����ĿΪ2NA | |
| B�� | ��״���£�22.4LH2O���еķ�����ΪNA | |
| C�� | 5.6gFe�����������ᷴӦʱ��ʧȥ�ĵ�����Ϊ0.3NA | |
| D�� | 0.5mol/L��MgCl2��Һ�У�����Cl-����ΪNA |

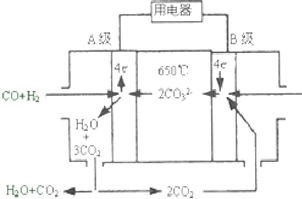 ����̼����ȼ�ϵ�أ�MCFS����������1889�꣮����һ��̼����ȼ�ϵ�أ���һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʣ������¶�Ϊ650�棬�ڴ��¶�������Ϊ��������ú����CO��H2�������Ϊ1��1��ֱ����ȼ�ϣ��乤��ԭ����ͼ��ʾ����ش��������⣺
����̼����ȼ�ϵ�أ�MCFS����������1889�꣮����һ��̼����ȼ�ϵ�أ���һ������Li2CO3��Na2CO3���ۻ����Ϊ����ʣ������¶�Ϊ650�棬�ڴ��¶�������Ϊ��������ú����CO��H2�������Ϊ1��1��ֱ����ȼ�ϣ��乤��ԭ����ͼ��ʾ����ش��������⣺