��Ŀ����
16���й����������վ������ʾ��PM2.5��SO2��NOx����������������Ӱ�����������������Ⱦ�����Ҫ��ԴΪȼú��������β���ȣ���ˣ������ǽ����о�������Ҫ���壮��ش���1����PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ�ȣ�mol/L�� | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��2��NOx������β������Ҫ��Ⱦ��֮һ����������������ʱ������N2��O2��Ӧ���������仯ʾ��ͼ��ͼ��
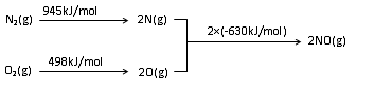
N2��g��+O2��g��?2NO ��g����H=+183kJ/mol��
���� ��1��������Һ�е���غ����H+����Ũ���Լ�����PH=-lgc��H+�����㣻
��2�����������仯ͼ���㷴Ӧ�ȣ���Ӧ��=��Ӧ��ļ��ܺ�-������ļ��ܺͣ�
��� �⣺��1����Һ�е���غ㣺C��K+��+C��NH4+��+c��Na+��+C��H+��=2C��SO42-��+C��NO3-��+C��Cl-����4��10-6mol/L+2��10-5mol/L+6��10-6mol/L+C��H+��=2��4��10-5mol/L+3��10-5mol/L+2��10-5mol/L����C��H+��=1��10-4mol•L-1��PH=-lgc��H+��=4������pHֵΪ4��
�ʴ�Ϊ��4��
��2���÷�Ӧ�еķ�Ӧ��=��Ӧ��ļ��ܺ�-������ļ��ܺ�=��945+498��kJ/mol-2��630kJ/mol=+183kJ/mol��
�ʴ�Ϊ��+183 kJ/mol��
���� ���⿼���˵������Һ�еĵ���غ㡢��Ӧ��=��Ӧ��ļ��ܺ�-������ļ��ܺ͵�֪ʶ��ע�ⷴӦ�ȵļ��㷽����Ϊ�״��㣬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
7������ʽΪC5H12O���к���2�����Ĵ������ʽΪC5H10O2���ᷢ��������Ӧ�õ����л�����ܵĽṹ�У������������칹����������
| A�� | 24�� | B�� | 16�� | C�� | 12�� | D�� | 8�� |
4����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӣ������Ϣ���±���ʾ�������ƶϻش��������⣺������ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��E�����ڱ���λ�ڵ������ڵ�IB�壬C��̬ԭ�Ӻ�������Ų�ʽ��1s22s22p63s1��
��2��A��B��D����Ԫ�ص縺���ɴ�С����˳��ΪN��C��Si������A������Ȼ���Ϊ�Ǽ��Է��ӣ�������Է��ӡ��Ǽ��Է��ӡ���
��3��A��B������⻯���зе�ϸߵ�NH3��ԭ����NH3���Ӽ���������
��4����֪��
��AH4��g��+2BO2��g��=B2��g��+AO2��g��+2H2O��g�� H1=-867kJ•mol-1
��2BO2��g��=B2 O4��g�� H2=-56.9kJ•mol-1
д��AH4��B2O4��Ӧ���Ȼ�ѧ����ʽCH4��g��+N2O4��g���TN2��g��+CO2��g��+2H2O��g����H=-810.1kJ/mol��
| A | A��һ�ֵ�������Ȼ����Ӳ����� |
| B | BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض��� |
| C | ͬ�����У�CԪ�ص�����������Ӧ��ˮ����ļ�����ǿ |
| D | D�Ļ�̬ԭ��M���������K���2 �� |
| E | E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ����� |
��2��A��B��D����Ԫ�ص縺���ɴ�С����˳��ΪN��C��Si������A������Ȼ���Ϊ�Ǽ��Է��ӣ�������Է��ӡ��Ǽ��Է��ӡ���
��3��A��B������⻯���зе�ϸߵ�NH3��ԭ����NH3���Ӽ���������
��4����֪��
��AH4��g��+2BO2��g��=B2��g��+AO2��g��+2H2O��g�� H1=-867kJ•mol-1
��2BO2��g��=B2 O4��g�� H2=-56.9kJ•mol-1
д��AH4��B2O4��Ӧ���Ȼ�ѧ����ʽCH4��g��+N2O4��g���TN2��g��+CO2��g��+2H2O��g����H=-810.1kJ/mol��
8������˵����ȷ���ǣ�������
| A�� | ��ϵͳ�������������� ������Ϊ2-��-2-�һ����� ������Ϊ2-��-2-�һ����� | |
| B�� | �ֱ���������Ӧ��ɺ����Һ��������ţ���м���Ũʳ��ˮ���й������� | |
| C�� | ̼ԭ����С��5����������4�֣�����֮�以Ϊͬϵ�� | |
| D�� | ʯ���ѽ���Եõ���ϩ��ú����õ���ú�����п�����ȡ������ϩ�ͱ�ʹ��ˮ��ɫ��ԭ����ͬ |
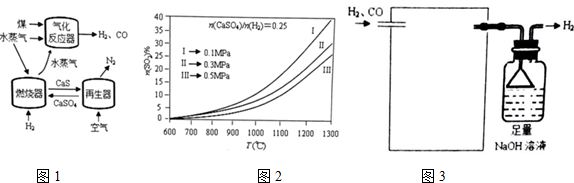
6����ҵ���ɽ�̿����Ȼ���������Ĺ����л����һ����̼��Ϊ�˳�ȥ�����л��е�һ����̼�����ڴ������ڵ������·�����Ӧ��
CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.0kJ/mol
�÷�Ӧ�ڹ�ҵ�ϱ���Ϊ��һ����̼�任����Ӧ��
��1����һ���ݻ�Ϊ1L���ܱ������м���1molCO��3molH2O��g��������������Ӧ��5min��ﵽƽ�⣬��ø÷�Ӧʵ�ʷų�������Ϊ20.5kJ��Ѹ�ٵ��������������������¶Ȳ��䣮���ʱ�䷶Χ�ڷ�Ӧ��ƽ�����ʦͣ�CO��=0.1mol/��L•min����ƽ�ⳣ��K����ֵΪ0.2��
��2��ij��ҵ�ϳɰ���ԭ�������Ϊ��H2 40%��N2 20%��CO 30%��CO210%����Ϊ�����������
�ֲ��á�һ����̼�任������������ԭ�����м���ˮ�����������е�CO��ȥ����֪��ͬ�¶�
����Ӧ��Ͷ�ϱȣ�$\frac{n��{H}_{2}O��}{n��CO��}$���£��任��Ӧ��ƽ����������CO������������±���ʾ��
��ƽ����������CO���������Խ����CO��ƽ��ת����ԽС�����С������
�ڴӸ÷�Ӧ�Ļ�ѧ����ʽ��������Ӧ��Ͷ�ϱȣ�$\frac{n��{H}_{2}O��}{n��CO��}$��Խ��CO��ƽ��ת����Խ����
����С���������ϱ��е����ݷ������������������������������ݶ��ϱ��ķ�����Ϊ���COƽ��ת���ʣ������Բ�ȡ�Ĵ�ʩ�ǽ����¶ȣ�
�۲�ͬ�¶��¸÷�ӦKֵ��K��300�棩��K��200�棩�����������=����������
���¶���һ����̼�任����������Ҫ��������ʵ�����������н��¶ȿ�����300�����ң���ԭ���������¶ȣ�����߷�Ӧ���ʣ���ƽ�������ƶ���CO��ƽ��ת�����½���ʵ��������Ӧ�ۺϿ������ʺ�ƽ���������森
CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.0kJ/mol
�÷�Ӧ�ڹ�ҵ�ϱ���Ϊ��һ����̼�任����Ӧ��
��1����һ���ݻ�Ϊ1L���ܱ������м���1molCO��3molH2O��g��������������Ӧ��5min��ﵽƽ�⣬��ø÷�Ӧʵ�ʷų�������Ϊ20.5kJ��Ѹ�ٵ��������������������¶Ȳ��䣮���ʱ�䷶Χ�ڷ�Ӧ��ƽ�����ʦͣ�CO��=0.1mol/��L•min����ƽ�ⳣ��K����ֵΪ0.2��
��2��ij��ҵ�ϳɰ���ԭ�������Ϊ��H2 40%��N2 20%��CO 30%��CO210%����Ϊ�����������
�ֲ��á�һ����̼�任������������ԭ�����м���ˮ�����������е�CO��ȥ����֪��ͬ�¶�
����Ӧ��Ͷ�ϱȣ�$\frac{n��{H}_{2}O��}{n��CO��}$���£��任��Ӧ��ƽ����������CO������������±���ʾ��
| �¶�/��\CO���������%\Ͷ�ϱ� | $\frac{n��{H}_{2}O��}{n��CO��}$=1 | $\frac{n��{H}_{2}O��}{n��CO��}$=3 | $\frac{n��{H}_{2}O��}{n��CO��}$=5 |
| 200 250 300 350 | 1.70 2.73 6.00 7.85 | 0.21 0.30 0.84 1.52 | 0.02 0.06 0.43 0.80 |
�ڴӸ÷�Ӧ�Ļ�ѧ����ʽ��������Ӧ��Ͷ�ϱȣ�$\frac{n��{H}_{2}O��}{n��CO��}$��Խ��CO��ƽ��ת����Խ����
����С���������ϱ��е����ݷ������������������������������ݶ��ϱ��ķ�����Ϊ���COƽ��ת���ʣ������Բ�ȡ�Ĵ�ʩ�ǽ����¶ȣ�
�۲�ͬ�¶��¸÷�ӦKֵ��K��300�棩��K��200�棩�����������=����������
���¶���һ����̼�任����������Ҫ��������ʵ�����������н��¶ȿ�����300�����ң���ԭ���������¶ȣ�����߷�Ӧ���ʣ���ƽ�������ƶ���CO��ƽ��ת�����½���ʵ��������Ӧ�ۺϿ������ʺ�ƽ���������森
7���������ӷ���ʽ��д��ȷ���ǣ�������
| A�� | ʯ̿����NaOH��Һ��ϣ�H++OH-��H2O | |
| B�� | ϡHNO3ϴ���Թ��е�������Ag+NO3-+2H+�TAg++NO��+H2O | |
| C�� | ��ϩʹ����KMnO4��ɫ��5C2H4+12MnO4-+36H+��12Mn2++10CO2��+28H2O | |
| D�� | ��CH2BrCOOH�м�������������������Һ�����ȣ� |
 ��
��