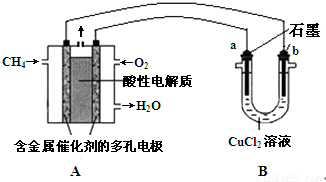
��Ŀ����
12��ij�л���A������ʽΪC4H8O2�����Ǿ���ˮ����ζ��Һ�壬������ˮ�����Է�����ͼ��ʾ��ת����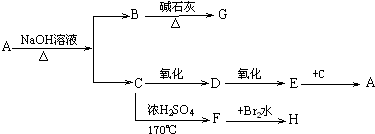
��1���ƶ��й����ʵ����ƣ�
A������������B�������ƣ�C���Ҵ���
D����ȩ��E�����ᣬF����ϩ��
��2��д����A����B��C�Ļ�ѧ����ʽCH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH
��3��д����C����D�Ļ�ѧ����ʽ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O
��4����֪B+NaOH��Na2CO3+CH4�� �÷�Ӧ��������ȡ����Ӧ��
���� ����B+NaOH��Na2CO3+CH4����֪BΪCH3COONa��ij�л���A������ʽΪC4H8O2�����Ǿ���ˮ����ζ�����壬������ˮ��˵��AΪ��������ˮ������B��C��C�ܾ����������������B�Ľṹ��ʽ��֪��AΪCH3COOCH2CH3��CΪCH3CH2OH���������и����ʵ�ת����ϵ�������Ƶ�DΪCH3CHO��EΪCH3COOH��FΪCH2=CH2��HΪBrCH2CH2Br���ݴ˴��⣮
��� �⣺����B+NaOH��Na2CO3+CH4����֪BΪCH3COONa��ij�л���A������ʽΪC4H8O2�����Ǿ���ˮ����ζ�����壬������ˮ��˵��AΪ��������ˮ������B��C��C�ܾ����������������B�Ľṹ��ʽ��֪��AΪCH3COOCH2CH3��CΪCH3CH2OH���������и����ʵ�ת����ϵ�������Ƶ�DΪCH3CHO��EΪCH3COOH��FΪCH2=CH2��HΪBrCH2CH2Br��
��1��������ķ�����֪��A������������B�������ƣ�C���Ҵ���D����ȩ��E�����ᣬF����ϩ��
�ʴ�Ϊ�����������������ƣ��Ҵ�����ȩ�������ϩ��
��2����A����B��C�Ļ�ѧ����ʽΪCH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH��
�ʴ�Ϊ��CH3COOCH2CH3+NaOH$\stackrel{��}{��}$CH3COONa+CH3CH2OH��
��3����C����D�Ļ�ѧ����ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��4��CH3COONa+NaOH��Na2CO3+CH4���У���Ӧ��������ȡ����Ӧ��
�ʴ�Ϊ��ȡ����Ӧ��
���� ���⿼�����л�����ƶϣ���ȷ�л���Ĺ����ż��������ǽⱾ��ؼ������ճ������л���Ӧԭ�����ѶȲ���

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�| A�� | ����������Һ��pH=4 | |
| B�� | ����NaOH��Һ������ĵ���ƽ�������ƶ���Kֵ���� | |
| C�� | ��������0.01 mol•L-1 NaOH��Һ��������Һ��pH=7 | |
| D�� | ��������0.01 mol•L-1 NaOH��Һ��������Һ��pH��7 |
| A�� | ��״���£�11.2 L�ļ��������ķ�����Ϊ0.5 NA �� | |
| B�� | 28 g��ϩ�������õ��Ӷ���ĿΪ6 NA�� | |
| C�� | ��״���£�11.2 L���ȼ�������������Ϊ0.5 NA�� | |
| D�� | ������ϩ����ϩ����ϩ�Ļ�����干14 g����ԭ����Ϊ6 NA�� |
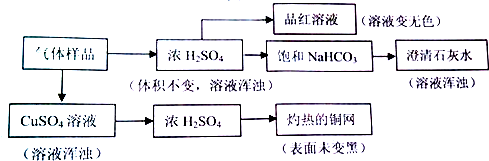
������ʵ�������ж���ȷ���ǣ�������
| A�� | ��ʹƷ����Һ��ɫ��˵��ԭ������Ʒ�����ٺ���Cl2��SO2 | |
| B�� | ���ȵ�ͭ��δ��ڣ�˵��ԭ������Ʒ��һ��������O2 | |
| C�� | ����ʯ��ˮ����ǣ�˵��ԭ������Ʒ�к���SO2������ȷ���Ƿ���CO2 | |
| D�� | ��������ͭ��Һ����Һ����ǣ�˵��ԭ������Ʒ�к���H2S������ų�SO2��Cl2�Ĵ��� |
| A�� | ��Ƭ��ϡ����ķ�Ӧ | B�� | Ba��OH��2��8H2O��NH4Cl�ķ�Ӧ | ||
| C�� | ���ȵ�̿��CO2�ķ�Ӧ | D�� | ϡ������ϡ����������Һ�ķ�Ӧ |
 ��
��