ЬтФПФкШн
ЁОЬтФПЁПФГЮТЖШЪБЃЌЫЎЕФРызгЛ§ГЃЪ§ Kw = 1ЁС10-13ЃЌНЋДЫЮТЖШЯТ pH = 11 ЕФ Ba(OH)2ШмвК a L гы pH =1ЕФ H2SO4 ШмвК b L ЛьКЯ(ЩшЛьКЯШмвКЬхЛ§ЮЊСНепжЎКЭЃЌЙЬЬхЬхЛ§КіТдВЛМЦ)ЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
A. Шє aЁУb = 9ЁУ2ЃЌдђЫљЕУШмвК pH ЕШгк 2
B. Шє aЁУb = 9ЁУ2ЃЌИУЛьКЯШмвКзюЖрФмШмНтЬњЗл 0.28(a + b) g
C. ШєЫљЕУЛьКЯШмвКЮЊжаадЃЌдђ aЁУb = 1ЁУ1
D. ШєЫљЕУЛьКЯШмвКЮЊжаадЃЌЩњГЩГСЕэЕФЮяжЪЕФСПЮЊ 0.05b mol
ЁОД№АИЁПC
ЁОНтЮіЁП
A.Шєa:b=9:2ЃЌЩшBa(OH)2ШмвКЬхЛ§ЮЊ9LЃЌH2SO4ШмвКЬхЛ§ЮЊ2LЁЃpH=11ЕФBa(OH)2ШмвКжа![]() ЃЌдђn(OH-)=10-2mol/LЁС9L=0.09molЁЃpH =1ЕФH2SO4ШмвКжаc(H+)=0.1mol/LЃЌдђn(H+)=0.1mol/LЁС2L=0.2molЃЌЫљвдn(H+)> n(OH-)ЃЌжаКЭЗДгІжаЫсЙ§СПЃЌЛьКЯКѓЫљЕУШмвКЯдЫсадЃЌЛьКЯШмвКжаH+ХЈЖШЮЊ
ЃЌдђn(OH-)=10-2mol/LЁС9L=0.09molЁЃpH =1ЕФH2SO4ШмвКжаc(H+)=0.1mol/LЃЌдђn(H+)=0.1mol/LЁС2L=0.2molЃЌЫљвдn(H+)> n(OH-)ЃЌжаКЭЗДгІжаЫсЙ§СПЃЌЛьКЯКѓЫљЕУШмвКЯдЫсадЃЌЛьКЯШмвКжаH+ХЈЖШЮЊ![]() ЃЌЦфЫљЕУШмвКpH=2ЃЌAЯюе§ШЗЃЛ
ЃЌЦфЫљЕУШмвКpH=2ЃЌAЯюе§ШЗЃЛ
B.гЩAЯюПЩжЊЃЌШєa:b=9:2ЫљЕУЛьКЯШмвКЕФpH=2ЃЌМДЛьКЯШмвКжаH+ХЈЖШЮЊ0.01mol/LЃЌЛьКЯШмвКЬхЛ§ЮЊ(a+b)LЃЌдђЛьКЯШмвКжаH+ЮяжЪЕФСПЮЊ0.01mol/LЁС(a+b)L=0.01(a+b)molЁЃШмНтЬњЗлЕФЗДгІЪНЮЊFe+2H+=Fe2++H2ЁќЃЌЫљвдШмНтЬњЗлЕФжЪСПЃК ![]() ЃЌBЯюе§ШЗЃЛ
ЃЌBЯюе§ШЗЃЛ
C.pH=11ЕФBa(OH)2ШмвКжа![]() ЃЌдђn(OH-)=10-2mol/LЁСaL=0.01amolЁЃpH =1ЕФH2SO4ШмвКжаc(H+)=0.1mol/LЃЌдђn(H+)=0.1mol/LЁСbL=0.1bmolЃЌШєЪЙЫљЕУЛьКЯШмвКЮЊжаадЃЌдђn(H+)= n(OH-)ЃЌМД0.1bmol =0.01amolЃЌa:b=10:1ЃЌCЯюДэЮѓЃЛ
ЃЌдђn(OH-)=10-2mol/LЁСaL=0.01amolЁЃpH =1ЕФH2SO4ШмвКжаc(H+)=0.1mol/LЃЌдђn(H+)=0.1mol/LЁСbL=0.1bmolЃЌШєЪЙЫљЕУЛьКЯШмвКЮЊжаадЃЌдђn(H+)= n(OH-)ЃЌМД0.1bmol =0.01amolЃЌa:b=10:1ЃЌCЯюДэЮѓЃЛ
D.ШєЫљЕУЛьКЯШмвКЮЊжаадЃЌдђЛьКЯЧАСђЫсжаH+КЭBa(OH)2жаOH-ЮяжЪЕФСПЯрЕШЃЌШЁЮЊ0.1bmolЃЌЛьКЯЧАn(H2SO4)=n[Ba(OH)2]=0.05bmolЃЌЛьКЯЪБH2SO4 +Ba(OH)2=BaSO4Ё§+2H2OЃЌЫљвдЩњГЩBaSO4ГСЕэЕФЮяжЪЕФСПЮЊ0.05bmolЃЌDЯюе§ШЗЃЛД№АИбЁCЁЃ

 ВНВНИпПкЫуЬтПЈЯЕСаД№АИ
ВНВНИпПкЫуЬтПЈЯЕСаД№АИ ЕуОІаТНЬВФШЋФмНтЖСЯЕСаД№АИ
ЕуОІаТНЬВФШЋФмНтЖСЯЕСаД№АИ аЁбЇНЬВФЭъШЋНтЖСЯЕСаД№АИ
аЁбЇНЬВФЭъШЋНтЖСЯЕСаД№АИЁОЬтФПЁПЯђЫФжЇЪдЙмжаЗжБ№МгШыЩйСПВЛЭЌЕФЮоЩЋШмвКНјааШчЯТВйзїЃЌНсТле§ШЗЕФЪЧ(ЁЁЁЁ)
бЁЯю | Вйзї | ЯжЯѓ | НсТл |
A | ЕЮМгBaCl2ШмвК | ЩњГЩАзЩЋГСЕэ | дШмвКжагаSO42ЁЊ |
B | ЯШЕЮМгзуСПЯЁЯѕЫсЃЌдйЕЮШыAgNO3ШмвК | ПЊЪМЮоУїЯдЯжЯѓЃЌКѓВњЩњАзЩЋГСЕэ | дШмвКжагаClЃ |
C | гУНрОЛВЌЫПеКШЁШмвКНјаабцЩЋЗДгІ | Л№бцГЪЛЦЩЋ | дШмвКжагаNaЃЋЃЌЮоKЃЋ |
D | ЕЮМгЯЁNaOHШмвКЃЌНЋЪЊШѓКьЩЋЪЏШяЪджНжУгкЪдЙмПк | ЪджНВЛБфРЖ | дШмвКжаЮоNH4+ |
A. AB. BC. CD. D
ЁОЬтФПЁПФГЪЕбщаЁзщЩшМЦгУ50 mL 0.50 molЁЄLЃ1бЮЫсИњ50 mL 0.55 molЁЄLЃ1ЧтбѕЛЏФЦШмвКдкШчЭМзАжУжаНјаажаКЭЗДгІЁЃЪдЛиД№ЯТСаЮЪЬтЃК
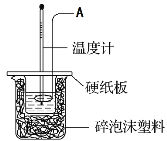
ЃЈ1ЃЉвЧЦїAЕФУћГЦ__________________________
ЃЈ2ЃЉЪЕбщЙ§ГЬжаЯТСаВйзїе§ШЗЪЧ______________(ЬюзжФИ)ЁЃ
A.гУЮТЖШМЦаЁаФНСАш
B.ЗжЖрДЮМгШыЧтбѕЛЏФЦШмвК
C.жЛНјаавЛДЮЪЕбщЃЌгУЫљЛёЕУЕФЪ§ОнМЦЫужаКЭШШ
D.гУЬздкЮТЖШМЦЩЯЕФAЧсЧсЕиЩЯЯТНСЖЏ
ЃЈ3ЃЉМйЩшбЮЫсКЭЧтбѕЛЏФЦШмвКЕФУмЖШЖМЪЧ1 gЁЄcmЃ3ЃЌгжжЊжаКЭЗДгІКѓЩњГЩШмвКЕФБШШШШнcЃН4.18 JЁЄgЃ1ЁЄЁцЃ1ЁЃЮЊСЫМЦЫужаКЭШШЃЌФГбЇЩњЪЕбщМЧТМЪ§ОнШчЯТЃК
ЪЕбщађКХ | Ц№ЪМЮТЖШt1/Ёц | жежЙЮТЖШt2/Ёц | |
бЮЫс | ЧтбѕЛЏФЦШмвК | ЛьКЯШмвК | |
1 | 20.0 | 20.1 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.5 | 20.6 | 23.6 |
вРОнИУбЇЩњЕФЪЕбщЪ§ОнМЦЫуЃЌИУЪЕбщВтЕУЕФжаКЭШШІЄHЃН__________ (НсЙћБЃСєвЛЮЛаЁЪ§)ЁЃ
ЃЈ4ЃЉвдЯТВйзїЃЌЛсЪЙВтЕУЕФжаКЭШШІЄHЗЂЩњдѕбљЕФБфЛЏЃП(ЬюЁАЦЋДѓЁБЁАЦЋаЁЁБЛђЁАВЛБфЁБ)ЁЃ
ЂйСПШЁЯЁбЮЫсЪБИЉЪгСПЭВЖСЪ§ЃЌВтЕУЕФжаКЭШШІЄHЛс_______________ ЁЃ
ЂкдкжаКЭШШВтЖЈЪЕбщжаЮДгУЫЎЯДЕгЮТЖШМЦЩЯЕФбЮЫсжБНгВтЖЈМюЕФЮТЖШЃЌВтЕУЕФжаКЭШШІЄHЛс_______________ЁЃ
ЂлШєгУЕШХЈЖШЕФДзЫсгы NaOH ШмвКЗДгІЃЌдђВтЕУЕФжаКЭШШІЄHЛс __________ЃЌЦфдвђЪЧ____________________________________________________________________________ЁЃ


