��Ŀ����
2��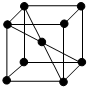 ��֪AΪ���ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B��C��D��Ϊ�ڶ�����Ԫ�أ�����B��C��D�ĵ縺�ԣ�D��C��B����һ�����ܣ�C��D��B����C�չ����E��Aͬ���壬��F��Gͬ���ڣ�FΪ�����ڽ���Ԫ�أ��䵥�ʼȿ��������ֿ�����Ӧ�ų�H2��G�ĵ����������û�пչ�����ҳɶԵ�����ռ�еĹ������δ�ɶԵ�����ռ�������3����
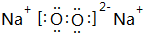
��֪AΪ���ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B��C��D��Ϊ�ڶ�����Ԫ�أ�����B��C��D�ĵ縺�ԣ�D��C��B����һ�����ܣ�C��D��B����C�չ����E��Aͬ���壬��F��Gͬ���ڣ�FΪ�����ڽ���Ԫ�أ��䵥�ʼȿ��������ֿ�����Ӧ�ų�H2��G�ĵ����������û�пչ�����ҳɶԵ�����ռ�еĹ������δ�ɶԵ�����ռ�������3������1��C2D�����е�����ԭ����N��B22-������Bԭ�ӵ��ӻ���ʽΪsp��
��2��E2D2�ĵ���ʽ��
 ������F2D3�����ʵ���֮��Ϊ1��1��Ϻ�Ͷ��ˮ�У���������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2Al2O3=4NaAlO2+O2����
������F2D3�����ʵ���֮��Ϊ1��1��Ϻ�Ͷ��ˮ�У���������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2Al2O3=4NaAlO2+O2������3��EABD3��Һ�У���������Ũ����С����Ĺ�ϵʽ��c��Na+����c��HCO3-����c��OH-����c��H+����c��CO32-��������F����Ϊ�缫���ϣ�������Һʱ���������ķ����ĵ缫��ӦʽΪAl-3e-+3HCO3-=Al��OH��3��+3CO2����
��4��һ������CD2��D2��CD�������Ͷ��ˮ��ǡ�ñ���ȫ���գ�����������C��Dԭ�ӵĸ�����Ϊ5��2��
��5��E���ʵľ�����ͼ����ѻ�ģ�������������ܶѻ�����λ����8��
��6����ҵ��ұ��F�ĵ���ʱ������F2D3Ϊԭ�϶�����FG3��ԭ�ϵ�ԭ����AlCl3�ǹ��ۻ�������ڷ��Ӿ��壬���Ȼ����������ڲ����磬�������ڵ�⣬��Al2O3�����ӻ���������ܵ��磮
���� AΪ���ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�B��C��D��Ϊ�ڶ�����Ԫ�أ�����B��C��D�ĵ縺�ԣ�D��C��B����һ�����ܣ�C��D��B����C�չ����Cֻ�ܴ���VA�壬��CΪNԪ�أ�DΪOԪ�أ�������⣨3����ΪNaHBO3��B�Ļ��ϼ�Ϊ+4����BΪ̼Ԫ�أ�FΪ�����ڽ���Ԫ�أ��䵥�ʼȿ��������ֿ�����Ӧ�ų�H2����FΪAl��E��Aͬ���壬��F��Gͬ���ڣ������ڵ������ڣ���EΪNa��G�ĵ����������û�пչ�����ҳɶԵ�����ռ�еĹ������δ�ɶԵ�����ռ�������3������������Ų�Ϊ1s22s22p63s23p5����GΪCl���ݴ˽��
��� �⣺AΪ���ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�B��C��D��Ϊ�ڶ�����Ԫ�أ�����B��C��D�ĵ縺�ԣ�D��C��B����һ�����ܣ�C��D��B����C�չ����Cֻ�ܴ���VA�壬��CΪNԪ�أ�DΪOԪ�أ�������⣨3����ΪNaHBO3��B�Ļ��ϼ�Ϊ+4����BΪ̼Ԫ�أ�FΪ�����ڽ���Ԫ�أ��䵥�ʼȿ��������ֿ�����Ӧ�ų�H2����FΪAl��E��Aͬ���壬��F��Gͬ���ڣ������ڵ������ڣ���EΪNa��G�ĵ����������û�пչ�����ҳɶԵ�����ռ�еĹ������δ�ɶԵ�����ռ�������3������������Ų�Ϊ1s22s22p63s23p5����GΪCl��
��1��N2O��CO2�����ǵȵ����壬������̼��Ц�����Ӿ������ƵĽṹ����N2O��������ԭ��ֻ��һ����ԭ������������Ц���ĵ���ʽΪ��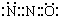 ��N2O�����е�����ԭ����N��C22-������Cԭ��֮���γ�̼̼������ÿ��Cԭ����1�Թ¶Ե��ӣ���Cԭ���ӻ������ĿΪ2����ȡsp�ӻ���
��N2O�����е�����ԭ����N��C22-������Cԭ��֮���γ�̼̼������ÿ��Cԭ����1�Թ¶Ե��ӣ���Cԭ���ӻ������ĿΪ2����ȡsp�ӻ���
�ʴ�Ϊ��N��sp��
��2��Na2O2�ĵ���ʽ�� ������Al2O3�����ʵ���֮��Ϊ1��1��Ϻ�Ͷ��ˮ�У�����������ˮ��Ӧ��������������������������������������Ӧ����ƫ��������ˮ���õ�����������������ǡ�÷�Ӧ����������Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2Al2O3=4NaAlO2+O2����
������Al2O3�����ʵ���֮��Ϊ1��1��Ϻ�Ͷ��ˮ�У�����������ˮ��Ӧ��������������������������������������Ӧ����ƫ��������ˮ���õ�����������������ǡ�÷�Ӧ����������Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2Al2O3=4NaAlO2+O2����
�ʴ�Ϊ�� ��2Na2O2+2Al2O3=4NaAlO2+O2����
��2Na2O2+2Al2O3=4NaAlO2+O2����
��3��NaHCO3��Һ�У�HCO3-��ˮ��̶ȴ��ڵ���̶ȣ���Һ�ʼ��ԣ���Һ��������Դ��ˮ�ĵ��뼰HCO3-�ĵ��룬����Һ����������Ũ����С����Ĺ�ϵʽ�ǣ�c��Na+����c��HCO3-����c��OH-����c��H+����c��CO32-��������Al����Ϊ�缫���ϣ����NaHCO3��Һ����������������Ӧ��Al�ŵ�õ�Al3+��Al3+����Һ��
HCO3-����ˮ�ⷴӦ����Al��OH��3��CO2���������ķ����ĵ缫��ӦʽΪ��Al-3e-+3HCO3-=Al��OH��3��+3CO2����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��OH-����c��H+����c��CO32-����Al-3e-+3HCO3-=Al��OH��3��+3CO2����
��4��һ������NO2��O2��NO�������Ͷ��ˮ��ǡ�ñ���ȫ���գ��������Ϸ�Ӧ����HNO3��Hԭ����Դ��ˮ��HNO3���Ը�дΪNO��3-0.5��.$\frac{1}{2}$H2O������������N��Oԭ�ӵĸ�����Ϊ1��2.5=5��2��
�ʴ�Ϊ��5��2��
��5��E���ʵľ�����ͼ ����ѻ�ģ�������������ܶѻ�����λ����8��
����ѻ�ģ�������������ܶѻ�����λ����8��
�ʴ�Ϊ�����������ܶѻ���8��
��6��AlCl3�ǹ��ۻ�������ڷ��Ӿ��壬���Ȼ����������ڲ����磬�������ڵ�⣬��Al2O3�����ӻ���������ܵ��磬��˳���Al2O3Ϊԭ�϶�����AlCl3��ԭ�ϣ�
�ʴ�Ϊ��AlCl3�ǹ��ۻ�������ڷ��Ӿ��壬���Ȼ����������ڲ����磬�������ڵ�⣬��Al2O3�����ӻ���������ܵ��磮
���� ���⿼�����ʽṹ��ϵ��ϵ�ۺ�Ӧ�ã���Ŀ�Ƚ��ۺϣ��漰���ӽṹ���ӻ���ʽ������ʽ����ѧ����ʽ��д������Ũ�ȴ�С�Ƚϡ���ѧ����ʽ�йؼ��㡢�����ṹ������ұ���ȣ���Ҫѧ���߱���ʵ�Ļ�������1����N2O��������ԭ���ж�Ϊ�״��㡢�ѵ㣬��4����ע�����÷���ʽ��д������𣬱��ⷽ��ʽ�ķ������Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| NaOH��Һ | ������Һ | ����Cu��OH��2����Һ | ������ | |
| A | �кͷ�Ӧ | - | �ܽ� | �������� |
| B | - | ������ | ���Ⱥ���ש��ɫ���� | �������� |
| C | ˮ�ⷴӦ | ������ | ���Ⱥ���ש��ɫ���� | - |
| D | ˮ�ⷴӦ | - | - | - |
��2����֪������������������¿�ת��Ϊ���ᣨC3H6O3����ȡ9g��������������Na��Ӧ��������2.24LH2����״��������ȡͬ��������ͬ���ʵ������Ҵ���Ӧ������0.1mol������֬��1.8gˮ��������Cu������ʱ�ɱ������ɱ�ͪ�ᣨ
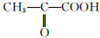 ������������ʵ��֪����Ľṹ��ʽΪCH3CH��OH��COOH��
������������ʵ��֪����Ľṹ��ʽΪCH3CH��OH��COOH����3���������������һ����������ˮ���ɻ�����C6H8O4������˻����Ľṹ��ʽ��
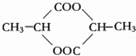 ��
��  �����ϩ���Ľṹʽ�����У�������
�����ϩ���Ľṹʽ�����У�������| A�� | 1 | B�� | 2 | C�� | 3 | D�� | 4 |
| A�� | 1.7g OH- ����������Ϊ0.9NA | |
| B�� | ��״���£�11.2 L HCl ����������Ϊ18NA | |
| C�� | 7.8gNa2O2�����к��е�������Ϊ4NA | |
| D�� | 0.5 mol D2O ������������Ϊ5NA |
| A�� | H2 | B�� | Cl2 | C�� | N2 | D�� | O2 |
| A�� | �����������������Ʋ����������Ʒ������ǰ���������� | |
| B�� | 2CaCO3��s��+2SO2��g��+O2��g���T2CaSO4��s��+2CO2��g���ڸ��������Է����У���÷�Ӧ�ġ�H��0 | |
| C�� | ���º����ܱ������н��еķ�ӦN2��g��+3H2��g��?2NH3��g������H=a kJ•mol-1��ƽ��ʱ���������ٳ���N2��H2����Ӧ���ʼӿ죬aֵ���� | |
| D�� | �����ᣨ���ᣩ�м�������CuSO4��Һ��H2S+CuSO4�TCuS��+H2SO4����H2S�ĵ���̶Ⱥ���Һ��pH������ |
| A�� | ��ϩ�ͱ�����ʹ��ˮ��ɫ����ɫ��ԭ����ͬ | |
| B�� | �����Cl2�ķ�Ӧ����ϩ��Br2�ķ�Ӧ����ͬһ���͵ķ�Ӧ | |
| C�� | �����Ǻ��ǵķ���ʽ��ΪC6H12O6������Ϊͬϵ�� | |
| D�� | �Ҵ������ᡢ�����������ܷ���ȡ����Ӧ�����������е�����������ñ���Na2CO3��Һ��ȥ |
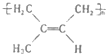 ����1mol�����1molBr2�����ӳɷ�Ӧ���ɵIJ�����3�֣�
����1mol�����1molBr2�����ӳɷ�Ӧ���ɵIJ�����3�֣�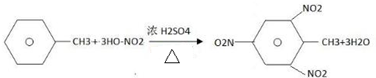 ��
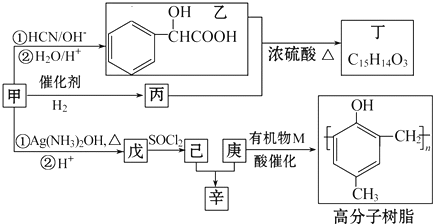
��

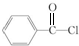 ����
����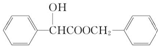 ��
��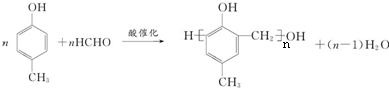 ��
��