��Ŀ����
����Ŀ���ߴ�������[Sr(NO3)2]�����������źŵơ���ѧ�����ȡ���ҵ�������Ⱥ�����ơ����ᱵ�����ʣ��ᴿ�������£�
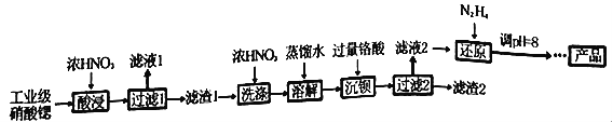
��֪��������Һ1������Ҫ������Ca(NO3)2��������1���ijɷ�ΪBa(NO3)2��Sr(NO3)2��������2������Ҫ�ɷ�Ϊ BaCrO4(���ʲ������ᷴӦ)���ڸ���(H2CrO4)Ϊ���ᡣ
��1������������ܲ��ø��µ�ԭ����_________________________________��
��2�������ˮϴ����ŨHNO3ϴ�ӵ��ŵ���_________________________________��
��3������Һ2���й�����H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ�����壬д����Ӧ�����ӷ���ʽ_______________________________________________________��
��4������Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s)![]() Cr3+(aq)+3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp=1.0��10��32����c(Cr3+)����1.0��10��5mol/L����ΪCr3+�Ѿ���ȫ�������ֽ���ԭ����Һ��pHֵ����4����ʱCr3+�Ƿ������ȫ?______________________(��ʽ����)��
Cr3+(aq)+3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp=1.0��10��32����c(Cr3+)����1.0��10��5mol/L����ΪCr3+�Ѿ���ȫ�������ֽ���ԭ����Һ��pHֵ����4����ʱCr3+�Ƿ������ȫ?______________________(��ʽ����)��
��5����֪Cr(OH)3����Al(OH)3����ԭ����Һ��pH���ܴ���8��ԭ���ǣ�___________��(������ӷ���ʽ˵������)��
��6��Ϊ�˲ⶨ������2���� BaCrO4�ĺ�������������ʵ�飺
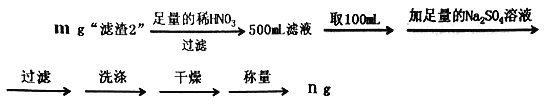
���ж�Ba2+��ȫ�����ķ�����____________________________________________��
��������2����BaCO4����������Ϊ______________________(�ô���ʽ��ʾ)��
���𰸡�����ŨHNO3�ӷ��ͷֽ� ����Sr(NO3)2�ܽ�������ʧ 4H2CrO4+3N2H4+12H+=4Cr3++3N2+16H2O c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ pH����8ʱCr(OH)3���ܽ���Cr(OH)3+ OH-=CrO2-+2H2O ���ã����ϲ���Һ�м����μ�Na2SO4��Һ���ϲ���Һ���ް�ɫ�������� ![]() ��100%
��100%
��������
��1��HNO3�ӷ��ͷֽ���
��2����������ŨHNO3�б���ˮ���ܽ��С��
��3��H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ������ΪN2������������ԭ��Ӧ����ʽ����ƽ��
��4������Ksp[Cr(OH)3]= c(Cr3+)![]() c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
��5����֪Cr(OH)3����Al(OH)3��Cr(OH)3�ڼ�����Һ�лᷢ��Cr(OH)3+ OH-=CrO2-+2H2O���ܽ⣻
��6���ٸ��������ƺ����ᱵ��Ӧ�������ᱵ������������з�����
�ڸ������ᱵ��������������2�����������м�����
��1��HNO3�ӷ��ͷֽ�������������ܲ��ø��µ�ԭ���DZ���HNO3�ӷ��ͷֽ���
�ʴ�Ϊ������HNO3�ӷ��ͷֽ���
��2������ͬ����ЧӦ�������ˮϴ����������ŨHNO3�б���ˮ���ܽ��С����ŨHNO3ϴ�ӵ��ŵ��Ǽ���������(������Ʒ��)�ܽ���ʧ��
�ʴ�Ϊ������Sr(NO3)2�ܽ�������ʧ��
��3������Һ2���й�����H2CrO4��N2H4��ԭΪCr3+��Cr�Ļ��ϼ۱仯��+6��+3������N�Ļ��ϼ����ߣ�ͬʱ����һ����ɫ��ζ�����壬Ϊ������N�Ļ��ϼ۱仯��-2��0����ת�Ƶ�����С������Ϊ��12�����ݵ�ʧ�����غ��ԭ���غ㣬���ӷ���ʽΪ��4H2CrO4+3N2H4+12H+=4Cr3++3N2+16H2O��
�ʴ�Ϊ��4H2CrO4+3N2H4+12H+=4Cr3++3N2+16H2O��
��4������Ksp[Cr(OH)3]= c(Cr3+)![]() c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
�ʴ�Ϊ��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
��5����֪Cr(OH)3����Al(OH)3��Cr(OH)3�ڼ�����Һ�лᷢ��Cr(OH)3+ OH-=CrO2-+2H2O���ܽ������Ի�ԭ����Һ��pH���ܴ���8��
�ʴ�Ϊ��pH����8ʱCr(OH)3���ܽ���Cr(OH)3+ OH-=CrO2-+2H2O��
��6�������ᱵҺ��Na2SO4��Һ��Ӧ�������ᱵ���Ȼ��ƣ������ж�Ba2+��ȫ�����ķ����ǣ����ã����ϲ���Һ�м����μ�Na2SO4��Һ���ϲ���Һ���ް�ɫ�������ɣ�
����������2����BaCrO4������Ϊx��
BaCrO4~~~BaSO4
253 233
X ng��5![]() =
=![]() �����x=
�����x=![]() g
g
����������2����BaCO4����������Ϊ:![]() ��100%=
��100%=![]() ��100%��
��100%��
�ʴ�Ϊ�����ã����ϲ���Һ�м����μ�Na2SO4��Һ���ϲ���Һ���ް�ɫ�������ɣ�![]() ��100%��
��100%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
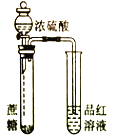
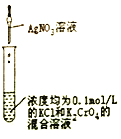
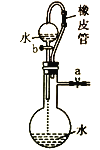
Сѧ��10����Ӧ����ϵ�д�����Ŀ�������й�ʵ��װ�á�����������ʵ����Ӧʵ��Ŀ�ĵ���
A | B | C | D | |
װ�� |
|
|
|
|
Ŀ�� | ��KOH��Һ��ȥ�屽�е����� | ֤��Ũ��������ˮ�ԡ�ǿ������ | �ȳ��ְ�ɫ�����������ש��ɫ������֤��Ksp(AgCl)< Ksp(Ag2CrO4) | ����ͨ���۲�ˮ�ܷ�ȫ���������ж�װ�������� |
A. A B. B C. C D. D
����Ŀ�����������ϵ����ȷ���ǣ� ��
ѡ�� | ԭ�� | ��� |
A | ����ֲ������ | ����ЧӦ |
B | SO2��NO2����Ĵ����ŷ� | ���� |
C | �������������ˮ�Ĵ����ŷ� | ˮ�����ೱ |
D | ����β���Ĵ����ŷ� | �⻯ѧ���� |
A. AB. BC. CD. D