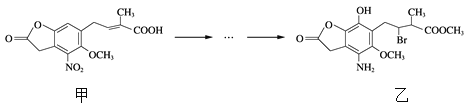
��Ŀ����
����Ŀ������Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李���������李��߰�ˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H��Ũ���ɴ�С��˳����(�����)___________��
��2���ܡ��ݡ��ޡ���������Һ��NH![]() Ũ���ɴ�С��˳����(�����)_______________��
Ũ���ɴ�С��˳����(�����)_______________��
��3�����ۺܰ͢������1��2��Ϻ��Һ�и�����Ũ���ɴ�С��˳���ǣ�__________________��
��4����֪t ��ʱ��KW��1��10��13����t ��(�����������������)________25�档��t ��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4��Һb L���(���Ի�Ϻ���Һ����ı仯)�������û����Һ��pH��2����a��b��________��
���𰸡� �ܢڢۢ� �ޢܢݢ� c(Cl��)>c(NH![]() )>c(Na��)>c(OH��)>c(H��) > 9��2
)>c(Na��)>c(OH��)>c(H��) > 9��2
�������������������1���Ȼ��Ϊǿ�������Σ�ˮ��ٽ�ˮ�ĵ��룬����Ϊ���ᣬ��Һ��������Ũ�Ƚ�С���������������Ϊǿ����ʣ�Ũ����ͬʱ�������ˮ�ĵ������Ƴ̶Ƚϴ�2���Ȼ�李�����李����������Һ�ж�����![]() ���Ȼ������ǿ����ʣ�����ȫ���룬������林��ڴ����������ӣ�����笠����ӵ�ˮ�⣬�����Ϊ����ǿ���Σ���ٽ�ˮ�⣬笠�����Ũ�����ˮΪ������ʣ����Ե��룬���笠�����Ũ����С����3����Ϻ���Һ����Ϊ�Ȼ��ƺ��Ȼ�李���ˮ���������غ��֪������Ũ�ȴ���笠�����Ũ�ȣ�������Һ�д���һˮ�ϰ������笠����Ӻ����������ӣ�һˮ�ϰ��ĵ���̶ȴ���笠�����ˮ��̶ȣ���笠�����Ũ�ȴ��������ӵ�Ũ�ȣ���Һ�ʼ��ԣ�������������Ũ�ȴ���������Ũ�ȣ���4��ˮ����Ϊ���ȹ��̣������¶ȴٽ����룬ˮ���ӻ�������������t������25����t��ʱ����������Ũ��
���Ȼ������ǿ����ʣ�����ȫ���룬������林��ڴ����������ӣ�����笠����ӵ�ˮ�⣬�����Ϊ����ǿ���Σ���ٽ�ˮ�⣬笠�����Ũ�����ˮΪ������ʣ����Ե��룬���笠�����Ũ����С����3����Ϻ���Һ����Ϊ�Ȼ��ƺ��Ȼ�李���ˮ���������غ��֪������Ũ�ȴ���笠�����Ũ�ȣ�������Һ�д���һˮ�ϰ������笠����Ӻ����������ӣ�һˮ�ϰ��ĵ���̶ȴ���笠�����ˮ��̶ȣ���笠�����Ũ�ȴ��������ӵ�Ũ�ȣ���Һ�ʼ��ԣ�������������Ũ�ȴ���������Ũ�ȣ���4��ˮ����Ϊ���ȹ��̣������¶ȴٽ����룬ˮ���ӻ�������������t������25����t��ʱ����������Ũ��![]() ��t��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4��Һb L��ϣ������û����Һ��pH��2����
��t��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4��Һb L��ϣ������û����Һ��pH��2����![]()

 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�����Ŀ��I.����β���dz��е���Ҫ������Ⱦ��о���������β����Ϊ������������Ҫ����������ȼ������ʱ������Ӧ��N2��g��+O2��g��![]() 2NO��g�����÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5 mol N2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��
2NO��g�����÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5 mol N2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��
��1��5 min�ڸ÷�Ӧ��ƽ������v��NO��=___________����T��ʱ���÷�Ӧ��ƽ�ⳣ��ֵΪ_________��
��2����Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯���ǣ�����ţ�___________��
a�����������ܶ� b����������ѹǿ
c������Ӧ���� d����λʱ���ڣ�N2��NO��������֮��
��3����֪������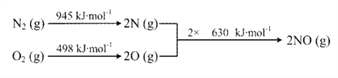
���� N2(g) + 2O2(g) === 2NO2(g) ��H= + 68 kJ��mol��1
����Ȼ�ѧ����ʽ��˵���¶ȶ���NO����NO2ƽ��ת���ʵ�Ӱ�죺_____________________��
II. ��pm2.5��������Ҫ�ɷ���SO2��NOx��CxHy������������ȡ�
��4����������������������_____________
��5��NaClO2��Һ��������SO2��NO����NaClO2��Һ��ͨ�뺬��SO2��NO�����壬��Ӧ�¶�Ϊ323 K��NaClO2��ҺŨ��Ϊ5��103mol��L1����Ӧһ��ʱ�����Һ������Ũ�ȵķ���������±�
���� | SO42 | SO32 | NO3 | NO2 | Cl |
c/��mol��L1�� | 8.35��104 | 6.87��106 | 1.5��104 | 1.2��105 | 3.4��103 |
��д��NaClO2��Һ������������Ҫ��Ӧ�����ӷ���ʽ________________________
����ѹǿ��NO��ת����______�����ߡ������䡱���͡�����
����ʵ������֪������Ӧ���ʴ���������Ӧ���ʣ���������������С��������ԭ�����SO2�ܽ��Դ���NO����������___________________
��6����ͼ���װ�ÿɽ�SO2��NOת��Ϊ(NH4)2SO4��
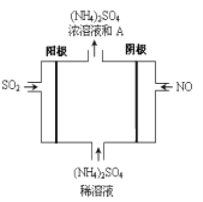
�������ĵ缫��Ӧʽ��______________________________
��SO2��NOͨ����װ���е������Ϊ___________________