��Ŀ����
��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽��������1����þ�Ͻ�
 �ⶨ���������ڱ�״���µ������V1L��
�ⶨ���������ڱ�״���µ������V1L������2����þ�Ͻ�
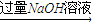 ��ַ�Ӧ��ⶨʣ������������W1g��
��ַ�Ӧ��ⶨʣ������������W1g������3����þ�Ͻ�
 ��Һ
��Һ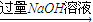 ���ˣ��ⶨ������������W2g��
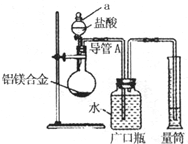
���ˣ��ⶨ������������W2g������ij��ѧʵ��С��������ͼ��ʾʵ��װ�ã����շ���1������ʵ�飬��ش��������⣺
��װ��������a��������______��
��ʵ��װ������һ�����Դ�����ָ��______��
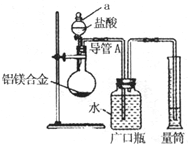
������2�з�����Ӧ�����ӷ���ʽΪ______��
������3�����ˡ��������õ��IJ�������������______��
��������3�������飬���þ����������Ϊ______��
������ijͬѧ�������������������ͬ�ķ���4��Ҳ�����þ���������������������������Ϻ��ʵ����ݣ����÷�������������
����4����þ�Ͻ�
 �ⶨ���������ڱ�״���µ������V2L����
�ⶨ���������ڱ�״���µ������V2L����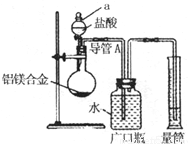
���𰸡�����������a�Ƿ�Һ©����
�ڹ��ƿ����ˮ���ܽ����Ӧ����Һ�����ϣ����ĵ���Ӧ����Һ�����£�
���������ܹ���ǿ����Һ��Ӧ����ƫ�����Σ�
�����ݲ������ڹ����е�������ɣ����ݳ�����������þ�����������þ�����ʵ���������þԭ���غ㣬������Ͻ���þ������������
����������������������������Һ��Ӧ���ɵ������������Ͻ���þ������������
����⣺�����ٸ���ͼʾ���ѿ���������a�����Ƿ�Һ©�����ʴ�Ϊ����Һ©����
�������Ǹ����ų���ˮ��������ⶨ���ɵ�������������˹��ƿ�еĽ�����Ӧ�ø�¶����Ƥ������������Ӧ���뵽ƿ�ף�
�ʴ�Ϊ�����ƿ�н������ܲ��뵽��ˮ�У���ˮ����δ�������ƿ�ײ���
������������������Һ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
���������ڹ����е����ã�����Һ���Ų��������£����������ã������������ƹ�����W2g������������þ�����ʵ����ǣ� mol��
mol��
þ����������Ϊ��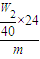 ×100%=
×100%=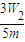 ×100%
×100%
�ʴ�Ϊ���������ã� ×100%��
×100%��
��������������������Һ�ͽ�������Ӧ�������������ݱ���������������������������ʵ����������þ������������
�ʴ�Ϊ����������������Һ��
���������⿼���˲ⶨþ���Ͻ���þ�����������������漰��ijЩ�����Ļ�ѧ���ʼ��йصĻ�ѧ����ʽ�ļ��㣬����ѧ���ķ������������������������Ѷ��еȣ�
�ڹ��ƿ����ˮ���ܽ����Ӧ����Һ�����ϣ����ĵ���Ӧ����Һ�����£�
���������ܹ���ǿ����Һ��Ӧ����ƫ�����Σ�
�����ݲ������ڹ����е�������ɣ����ݳ�����������þ�����������þ�����ʵ���������þԭ���غ㣬������Ͻ���þ������������
����������������������������Һ��Ӧ���ɵ������������Ͻ���þ������������
����⣺�����ٸ���ͼʾ���ѿ���������a�����Ƿ�Һ©�����ʴ�Ϊ����Һ©����
�������Ǹ����ų���ˮ��������ⶨ���ɵ�������������˹��ƿ�еĽ�����Ӧ�ø�¶����Ƥ������������Ӧ���뵽ƿ�ף�
�ʴ�Ϊ�����ƿ�н������ܲ��뵽��ˮ�У���ˮ����δ�������ƿ�ײ���
������������������Һ��Ӧ�����ӷ���ʽ��2Al+2OH-+2H2O�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O�T2AlO2-+3H2����
���������ڹ����е����ã�����Һ���Ų��������£����������ã������������ƹ�����W2g������������þ�����ʵ����ǣ�
 mol��
mol��þ����������Ϊ��
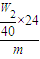 ×100%=
×100%=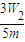 ×100%
×100%�ʴ�Ϊ���������ã�
 ×100%��
×100%����������������������Һ�ͽ�������Ӧ�������������ݱ���������������������������ʵ����������þ������������
�ʴ�Ϊ����������������Һ��
���������⿼���˲ⶨþ���Ͻ���þ�����������������漰��ijЩ�����Ļ�ѧ���ʼ��йصĻ�ѧ����ʽ�ļ��㣬����ѧ���ķ������������������������Ѷ��еȣ�

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ
 ��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽����
��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽����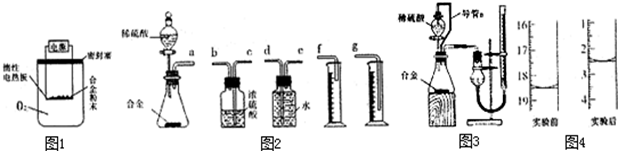
 ��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽����
��þ3%��5%����þ�Ͻ����ִ����졢������������е�������ҵ����Ҫ���ϣ�����һ������Ϊm g����þ�Ͻ����ⶨ����þ��������������λͬѧ����˲�ͬ��ʵ�鷽����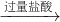 �ⶨ���������ڱ�״���µ������V1L��
�ⶨ���������ڱ�״���µ������V1L�� ��ַ�Ӧ��ⶨʣ������������W1g��
��ַ�Ӧ��ⶨʣ������������W1g�� �ⶨ���������ڱ�״���µ������V2L����
�ⶨ���������ڱ�״���µ������V2L����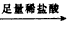 �ⶨ����������������״����
�ⶨ����������������״����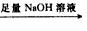 �ⶨ����������������״����
�ⶨ����������������״����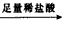 ��Һ
��Һ ���ˣ��ⶨ����������
���ˣ��ⶨ����������