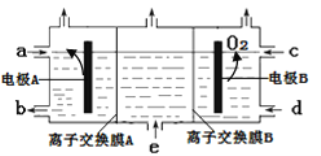
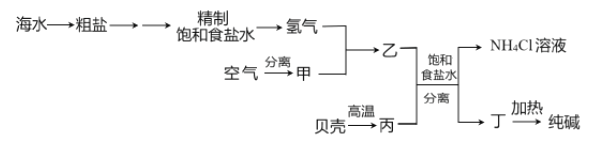
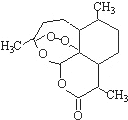
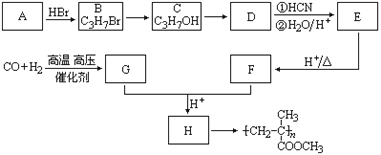
��Ŀ����
����Ŀ��������һ������A��һ�ֵ�ϩ��B��ɻ�����壬A��B�������ֻ����4��̼ԭ�ӣ���B���ӵ�̼ԭ������A���ӵĶࡣ
��1�����û������1L���ȼ����ͬ�¡�ͬѹ�µ�2.5LCO2�����ƶ�ԭ�������A�к�B���п��ܵ���ϼ��������___��
��2��120��ʱȡ1L�û��������9L������ϣ����ȼ�պ��ָ���120���ȼ��ǰ��ѹǿʱ���������6.25%����ͨ������ȷ��������и��ɷֵķ���ʽ___��
���𰸡�
��ϱ�� | A�ķ���ʽ | B�ķ���ʽ | A��B������ȣ�VA��VB�� |
�� | CH4 | C3H6 | 1��3 |
�� | CH4 | C4H8 | 1��1 |
�� | C2H6 | C3H6 | 1��1 |
�� | C2H6 | C4H8 | 3��1 |
C2H6��C4H8
��������
1�����������ȼ�պ�����2.5��CO2����B���ӵ�̼ԭ������A���ӵĶ࣬�������ֻ����̼ԭ����С��2.5��������CH4��C2H6����̼ԭ��������2.5��ϩ����C3H6��C4H8����ɡ�
��1���������ֻ����̼ԭ����С��2.5��������CH4��C2H6����̼ԭ��������2.5��ϩ����C3H6��C4H8����ɡ����������ֿ��ܵ���ϣ���CH4��C3H6����CH4��C4H8����C2H6��C3H6����C2H6��C4H8������ÿһ�������������ϩ����̼ԭ������ȼ�պ����ɵ�CO2�������ȷ��A��B������ȣ��磺��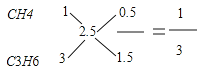 ����V��CH4����V��C3H6��=1��3��ͬ���ɵâ�CH4��C4H8�ı�1��1����C2H6��C3H6�ı���1��1����C2H6��C4H8�ı���3��1��
����V��CH4����V��C3H6��=1��3��ͬ���ɵâ�CH4��C4H8�ı�1��1����C2H6��C3H6�ı���1��1����C2H6��C4H8�ı���3��1��
��2����1����̬���������ȼ�պ�����仯Ϊ��V������CH4+2O2![]() CO2+2H2O��������V1=0��������C2H6+7/2 O2
CO2+2H2O��������V1=0��������C2H6+7/2 O2![]() 2CO2+3H2O��������V2=0.5��������C3H6+9/2 O2
2CO2+3H2O��������V2=0.5��������C3H6+9/2 O2![]() 3CO2+3H2O��������V3=0.5�������� C4H8+6O2
3CO2+3H2O��������V3=0.5�������� C4H8+6O2![]() 4CO2+4H2O��������V4=1.0��������������ϵ�1������������������ȼ�գ��������Ϊ����Ϣ�(��V1+3��V3)��4=0.375����������
4CO2+4H2O��������V4=1.0��������������ϵ�1������������������ȼ�գ��������Ϊ����Ϣ�(��V1+3��V3)��4=0.375����������![]() ������Ϣ�(��V1+��V4)��2=0.5����������
������Ϣ�(��V1+��V4)��2=0.5����������![]() ����Ϣ�(��V2+��V3)��2=0.5����������
����Ϣ�(��V2+��V3)��2=0.5����������![]() ����Ϣ�(3��V2+��V4)��4=0.625����������
����Ϣ�(3��V2+��V4)��4=0.625����������![]() ������Ϣܷ������⣬��AΪC2H6��BΪC4H8��
������Ϣܷ������⣬��AΪC2H6��BΪC4H8��

����Ŀ����������ṩ���Լ��ͷ�����ȥ����ĩ״������е�����(������Ϊ����)������ѡ�𰸵ı�����ڱ�����Ӧ�Ŀո��ڡ�
�ɹ�ѡ����Լ���A.���ᡡB.NaOH��Һ��C.O2��D.ˮ��E.���������Լ�
��ѡ�õIJ�������ˮϴ���ڼ��ȡ��۹��ˡ��ܽᾧ
��ĩ״����� | ��ѡ�Լ� | ��ѡ���� | |
(1) | CaCO3(SiO2) | ______ | ______ |
(2) | NaCl(SiO2) | ______ | ______ |
(3) | SiO2(Fe2O3) | ______ | ______ |
(4) | SiO2(CaCO3) | ______ | ______ |
(5) | SiO2(Si) | ______ | ______ |
(6) | SiO2(H2SiO3) | ______ | ______ |