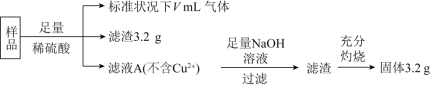��Ŀ����
����Ŀ��������������������ͷ�չ����Ҫ���ʻ������ش��������⣺
(1)�������������ܻ�ȡ�������ǽ������ϵ��� ______ (�����)��
�ٲ��ü���Ϊԭ�ϣ������������������������ʯ
�ڲ��ô�ͳ�����ý�̿��ԭSiO2�Ʊ�������
����ˮ�����������м����¯��������������;��ˮ��
���ڲ������������м���K��Pb��������������;�Ĺ�ѧ����
(2)ij������¯��һ��Ͷ�������ұ�����������ù�����ת�Ƶĵ���Ϊ1.60��105mol�������������ķ�Ӧ��Ҳ�����������е����ʣ������õ�����Ϊ ______t�������к�̼�����Ԫ�ؽ϶࣬������������Ϊԭ�ϣ���ȥ�����̼���������Ԫ�أ����г�ȥ��Ļ�ѧ����ʽΪ ______��
(3)��ҵ������Al�Ĺ����������£�
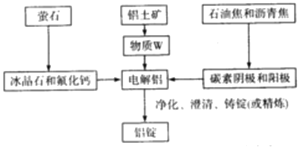
�ù����б���ʯ�ͷ����Ƶ������� ______������W�Ļ�ѧʽΪ ______ ����������Ĵ���Ϊ60%����ȡW����ʧ��Ϊ3%�����ʱ��ʧ��Ϊ0.1%����������ʱ����ʧ��Ϊ2%�����������ĺ���Ϊ99.9%����ô1.0t��������Ʊ����� ______t��
(4)������ʴ��������ʧ��������������ʴ��Ϊ������ʴ����������ӦʽΪ ______������������ʩ�ܶ࣬������������(����Zn)������������������������ӦʽΪ ______��
���𰸡��٢� 3.36 FeS+CaO![]() FeO+CaS ����Al2O3���۵㣬�������ڵ���ʵ��ܺ� Al2O3 0.3 O2+2H2O+4e-=4OH- Zn-2e-=Zn2+
FeO+CaS ����Al2O3���۵㣬�������ڵ���ʵ��ܺ� Al2O3 0.3 O2+2H2O+4e-=4OH- Zn-2e-=Zn2+
��������
(1)��ͳ���ǽ��������ǹ�ҵ�ͻ�������������Ļ������ϣ��粣����ˮ��ȣ��������ǽ���������20���������Ժ�չ�����ģ������������ܺ���;�IJ��ϣ��������ִ��¼������²�ҵ����ͳ��ҵ�������졢�ִ�����������ҽѧ������ȱ�ٵ����ʻ������ݴ˽����жϣ�
(2)���������Ҫ�ɷ�Ϊ���������������ݵ����غ��ȼ���������������ʵ������ټ��������������������������д���������������Ʒ�Ӧ�Ļ�ѧ����ʽ��
(3)����ʯ���ܽ�Al2O3���壬���������ۼ�����Al2O3���۵㣻WΪ���������Ҫ�ɷ����������������������������Ȼ��������Ԫ�������غ���ʽ���㣻
(4)��������(����Zn)����������������������пʧȥ��������п���ӣ��ݴ�д���缫��Ӧʽ��
(1)�ٲ��ü���Ϊԭ�ϣ����������������������Ľ��ʯΪ�������ǽ������ϣ�����ȷ��
�ڲ��ô�ͳ�����ý�̿��ԭSiO2�Ʊ��ĵ�����Ϊ�������ǽ������ϣ�����ȷ��
��ˮ���Ǵ�ͳ���ǽ������ϣ��۴���
�ܹ�ѧ�����Ǵ�ͳ���ǽ������ϣ��ܴ���
�ʺ���ѡ���Ǣ٢ڣ�
(2)������������ѧʽΪFe3O4��������Ԫ�ص�ƽ�����ϼ�Ϊ+![]() ���ù�����ת�Ƶĵ���Ϊ1.60��105mol��������Fe�����ʵ���n(Fe)=
���ù�����ת�Ƶĵ���Ϊ1.60��105mol��������Fe�����ʵ���n(Fe)=  =6��104mol�����Եõ�����������Ϊ��m=56g/mol��6��104mol=3.36��106g=3.36t��ͨ����CaO��ȥ��Ӧ��ѧ����ʽΪ��FeS+CaO
=6��104mol�����Եõ�����������Ϊ��m=56g/mol��6��104mol=3.36��106g=3.36t��ͨ����CaO��ȥ��Ӧ��ѧ����ʽΪ��FeS+CaO![]() FeO+CaS��
FeO+CaS��
(3)Al2O3�������Ӿ��壬�۵�ϸߣ��������ʯ�ͷ����ƣ����ڵı���ʯ�ͷ��������ܽ�Al2O3���壬�������ڵ��ұ����ʱ���������ۼ������������������ۻ�ʱ������¶ȣ��ڽ�������ұ���м������ʯ�ͷ����Ƶ�Ŀ���ǽ���Al2O3���۵㣬�������ڵ���ʵ��ܺģ�W������������Ҫ�ɷ�����������ѧʽΪAl2O3��
�����ɵ�����������Ϊx��������Ԫ�������غ�ɵã�1.0t��60%��![]() ��(1-3%)��(1-0.1%)��(1-2%)=99.9%x����ã�x=0.3t��
��(1-3%)��(1-0.1%)��(1-2%)=99.9%x����ã�x=0.3t��
(4)��������ʴ�У������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��O2+2H2O+4e-=4OH-���ڽ���������ʩ�У���������(����Zn)������Ϊԭ��ص�������������ԭ��Ӧ����������(����Zn)Ϊ������Znʧȥ��������Zn2+���缫��ӦʽΪ��Zn-2e-=Zn2+��

����Ŀ��ij��ѧѧϰС���������ʵ�鷽�����ⶨij����NaCl��С�մ���Ʒ��![]() ������������
������������
������һ�������ϣ�NaCl������![]() ʱ�ۻ������ֽ⣬
ʱ�ۻ������ֽ⣬![]() ���ȷֽ⣬
���ȷֽ⣬![]() ���ɴ����ʵ�飺�õ�����ƽ��ȡ
���ɴ����ʵ�飺�õ�����ƽ��ȡ![]() ��Ʒ�������������þƾ��Ƽ��ȣ���ͼ�������¶ȸ���
��Ʒ�������������þƾ��Ƽ��ȣ���ͼ�������¶ȸ���![]() ��������
��������![]() ���������غ���ȴ������ʣ���������Ϊ
���������غ���ȴ������ʣ���������Ϊ![]() ��
��
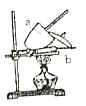
��1��ʵ�������ٳ���____�Ρ�
��2��ʵ���У��ﵽ���ز����ı���__________��
����������������![]() ��Һ�ʼ��ԣ�
��Һ�ʼ��ԣ�![]() �����������ʵ�飺ȷ��ȡ1.000 g��Ʒ��������ƿ���100 mL��Һ���õζ�����ȡ20.00 mL����ƿ�У�����2�μ���Ϊָʾ������0.1000mol/L�������Һ�ζ���ƽ�����ݣ�����ʵ����������£�
�����������ʵ�飺ȷ��ȡ1.000 g��Ʒ��������ƿ���100 mL��Һ���õζ�����ȡ20.00 mL����ƿ�У�����2�μ���Ϊָʾ������0.1000mol/L�������Һ�ζ���ƽ�����ݣ�����ʵ����������£�
1 | 2 | |
| 20.00 | 20.00 |
| 0.00 | 0.20 |
| 19.98 | 20.22 |
��3��ʵ���У���������������ȷʱ�����в�����������ʵ����������______����
A������ƿ������ˮϴ����ƿ����ˮ������ֱ��������Һ
B���ζ����ڱ���ˮ���װ���Һ
C����ƿ�ڱ���ˮ�飬�ô���Һ��ϴ����ʹ��
D����ƿ������ˮϴ����ֱ�ӷ������Һ���вⶨ
span>��4���ζ��յ���жϣ�__________��
��5������ʵ���������������ƽ��ֵΪ__________mL��
��6����Ʒ��![]() ����������__________��
����������__________��