��Ŀ����
3��Fridel-Crafts��Ӧ�����������������Ҫ�ķ������ںϳ����кܴ��ʵ�ü�ֵ���÷�Ӧ���Լ�ʾ���£�ArH+RX$\stackrel{����}{��}$ArR+HX����H��0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�������ᷴӦ�Ƶ��嶡���ȣ��е�50.7�棩��������Fridel-Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�99�棩����Ӧ���̼�ʵ��װ����ͼ1��ʾ��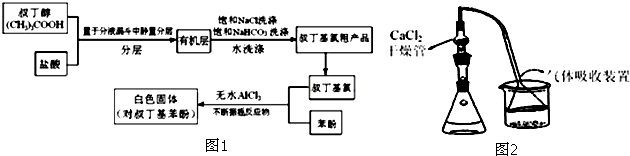
�Իش��������⣺
��1���������ֱ�ӽ����ᡢ�嶡��ֱ�ڷ�Һ©���У���ҡһ��ʱ����÷�Һ����ҡ������Ӧ���з�����������������ñ���ʳ��ˮ������̼�����Ƶ�Ŀ���dz�ȥ�嶡���ȴֲ����е�HCl���������ʡ������嶡�����ܽ���������������Ҫ���嶡���ȴֲ�Ʒ�Ƴɾ�Ʒ��ʵ���������������
��2�����������ͼ2��ʾ��װ���н��У���ʵ�������ȥ���Ʊ�װ����װ���Ȼ��Ƶĸ���ܣ��п��ܵ��µIJ��������ˮ�����룬��Ӧ������ȿ���ʧЧ��
��3���嶡�����뱽�η�Ӧʱ�ʵ������¶��Ǻ���Ҫ�ģ�����Ӧ�������¶ȹ���Ӧ����ˮԡ��ȴ��������ܵ��µIJ�������Ƿ�Ӧ����ʹ�¶����ߣ������ɴ�����HCl���壬������ʱ��ȴ���嶡���ȵ��������ݳ���Ӱ����ʣ�
��4���������嶡�����ױ��ʣ���ƿ��ʱƿ�ڻ��а���������ʵ�������õı����������䣬��д���嶡�����ڳ����±���ʱ������Ӧ�Ļ�ѧ����ʽ��CH3��3CCl��CH2=C��CH3��2+HCl����CH3��3CCl+H2O����CH3��3COH+HCl����
��5��ʵ���еõ���Ʒ�嶡�����γ������ǰ�ɫ������dz��ɫ������Ϊ���ܵ�ԭ����һ���ֱ��ӱ������е�������������
���� ��1�����ݷ�Һ©����ʹ��ע��������⣬�嶡���ȴֲ�������HCl�����ʣ��嶡������ʳ��ˮ������̼�������е��ܽ��С���ݴ˴��⣬�����嶡���ȵķе�ϵͣ����������룻
��2���Ȼ��Ƹ���ܾ�����ˮ���ã��ɷ�ֹ�Ȼ���ˮ������ʣ�
��3������Ӧ�������¶ȹ��ߣ��ɵ����嶡���Ȼӷ���
��4���������嶡�����ױ��ʣ���ƿ��ʱƿ�ڻ��а������������Ȼ�������������嶡���ȷ�����ȥ��Ӧ����CH2=C��CH3��2��HCl��CH3��3CClˮ������Ȼ��⣻
��5�������ױ����������ʣ�
��� �⣺��1��ʹ�÷�Һ©��ʱ����ҡ������Ӧ���з������嶡���ȴֲ�������HCl�����ʣ��嶡������ʳ��ˮ������̼�������е��ܽ��С�����Բ�������ñ���ʳ��ˮ������̼�����Ƶ�Ŀ���dz�ȥ�嶡���ȴֲ����е�HCl���������ʡ������嶡�����ܽ�������������嶡���ȵķе�ϵͣ����������룬
�ʴ�Ϊ����������ȥ�嶡���ȴֲ����е�HCl���������ʡ������嶡�����ܽ������������
��2���Ȼ��Ƹ���ܾ�����ˮ���ã��ɷ�ֹˮ�����룬ʹ��Ӧ�������ʧЧ��
�ʴ�Ϊ��ˮ�����룬��Ӧ������ȿ���ʧЧ��
��3����ӦΪ���ȷ�Ӧ�����ŷ�Ӧ�Ľ��У��¶������ߣ����ɴ�����HCl��������·�Ӧװ����ѹǿ���ߣ��ҿɵ����嶡���Ȼӷ���Ӱ����ʣ�
�ʴ�Ϊ����Ӧ����ʹ�¶����ߣ������ɴ�����HCl���壬������ʱ��ȴ���嶡���ȵ��������ݳ���Ӱ����ʣ�
��4���������嶡�����ױ��ʣ���ƿ��ʱƿ�ڻ��а������������Ȼ�������������嶡���ȷ�����ȥ��Ӧ����CH2=C��CH3��2��HCl��CH3��3CClˮ������Ȼ��⣬��Ӧ�ķ���ʽΪ��CH3��3CCl��CH2=C��CH3��2+HCl����CH3��3CCl+H2O����CH3��3COH+HCl����
�ʴ�Ϊ����CH3��3CCl��CH2=C��CH3��2+HCl����CH3��3CCl+H2O����CH3��3COH+HCl����
��5�������ױ��������ɶԱ������ɵ������ղ�Ʒ���嶡�����Ӳ��ǰ�ɫ��������ɫ���ʴ�Ϊ��һ���ֱ��ӱ������е�������������
���� �������л���ĺϳ�Ϊ�����ۺϿ��黯ѧ�Ʊ�ʵ�鷽������ƣ�������ѧ����ʵ�����������������ͷ��������Ŀ��飬Ϊ�߿��������ͣ��Ѷ��еȣ�ע�����ʵ�����ԭ���ͷ�����

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�| A�� | ��������A��E��B��A��D��C | B�� | ��������C��D��E��E��A | ||
| C�� | ��������A��E��F��E��B��D��C | D�� | ��������B��E��A��D��C |
| A�� | 0.1mol•L-1ϡ����100mL�к������������Ϊ0.1NA | |
| B�� | 1mol CH3+��̼�����ӣ��к��е�����Ϊ10NA | |
| C�� | 2.4g����þ�����������ᷴӦ��ת�Ƶ�����Ϊ2NA | |
| D�� | 12.4g�����к�����ԭ����ΪO.4NA |

������ϣ�
�������ܽ��
| ���� | KMnO4 | K2CO3 | KHCO3 | K2SO4 | CH3COOK |
| 20���ܽ�� | 6.4 | 111 | 33.7 | 11.1 | 217 |
�����״��ī��ɫ�ᾧ����ˮ��Һ������ɫ�������������MnO42-����������ɫ��
��ѧ���ʣ���ǿ������Һ���ȶ��������ԡ����Ժ������Ի����£�MnO42-�ᷢ���绯��Ӧ��
�Իش��������⣺
��1���������̿��KOH����ʱ��������ʯӢ������ѡ����������ʵ���������������ձ�¶�ڿ����еĹ����������Ӧ�Ļ�ѧ����ʽΪ2MnO2+4KOH+O2$\frac{\underline{\;����\;}}{\;}$2K2MnO4+2H2O��
��2��ʵ��ʱ����CO2����������KHCO3���������ӷ���ʽ��ʾʵ����ͨ������CO2ʱ������ϵ��KMnO4��Ʒ���Ƚ��͵�ԭ��3MnO42-+2CO2�T2MnO4-+MnO2��+2CO32-��
��3����ҵ��һ����ö��Ե缫����������Һ��ȡ������أ���д���õ�ⷴӦ�Ļ�ѧ����ʽ2K2MnO4+2H2O$\frac{\underline{\;���\;}}{\;}$2KMnO4+H2��+2KOH��
��ͳ���ղ�����Ĥ��ⷨ���ڸ���Ӧ������MnԪ�������ʺ͵���Ч�ʶ���ƫ�ͣ���ͬѧ���뵽���ӽ���Ĥ����ⱥ��ʳ��ˮ����Ľ����������������ӽ���Ĥ�ָ����������е�⣨��ͼ2����ͼ��A�ڼ������Һ���ΪKOH��Һ��ʹ�������ӽ���Ĥ�������MnԪ�������ʵ�ԭ��Ϊ�����ӽ���Ĥ��ֹ�������������������ԭ��
| A�� | �ɷǽ���Ԫ���γɵĻ�����һ���������ӻ����� | |
| B�� | ���н���Ԫ�صĻ�����һ�������ӻ����� | |
| C�� | ���ý�������÷ǽ�������ʱ�����γ����Ӽ� | |
| D�� | ��IA���VIIA��ԭ�ӻ���ʱ��һ���������Ӽ� |
| A�� | pH=2��HA��Һ��pH=12��MOH��Һ�������Ϻ�pH��7����HAΪ���� | |
| B�� | pH��ȵ�CH3COONa��NaOH��Na2CO3������Һ��c��NaOH����c��Na2CO3����c��CH3COONa�� | |
| C�� | ���ʵ���Ũ�Ⱦ�Ϊ0.2mol•L-1CH3COOH��CH3COONa��Һ�������ϣ�2c��Na+��=c��CH3COO-��+c��CH3COOH��=0.2mol•L-1 | |
| D�� | 0.1mol•L-1��NaHA��Һ����pH=4��c��HA-����c��H+����c��H2A����c��A2-�� |
 ɽ���ӣ�Kaempferol���ṹ��ͼ��ʾ���Ҵ���������ˮ�����߲ˡ����ࡢ��Ҷ�У����ж�������ѧ���ã��翹��������������������������ϸ�������ã��й�ɽ���ӵ�������ȷ���ǣ�������
ɽ���ӣ�Kaempferol���ṹ��ͼ��ʾ���Ҵ���������ˮ�����߲ˡ����ࡢ��Ҷ�У����ж�������ѧ���ã��翹��������������������������ϸ�������ã��й�ɽ���ӵ�������ȷ���ǣ�������| A�� | �ṹʽ�к���2���������ǻ����Ѽ����Ȼ���̼̼˫�� | |
| B�� | �ɷ���ȡ����Ӧ��ˮ�ⷴӦ���ӳɷ�Ӧ | |
| C�� | ����NaOH��Ӧ��������NaHCO3��Ӧ | |
| D�� | 1molɽ��������ˮ��Ӧ��������4molBr2 |
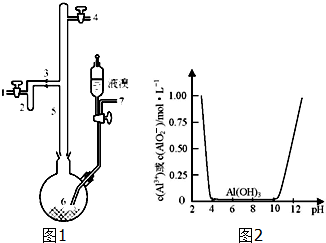 ���÷���������Ҫ�ɷ�ΪAl��������Fe��Si�ȣ��ȿ���ȡ�л��ϳɴ���AlBr3�ֿ���ȡ��ˮ�����������壮
���÷���������Ҫ�ɷ�ΪAl��������Fe��Si�ȣ��ȿ���ȡ�л��ϳɴ���AlBr3�ֿ���ȡ��ˮ�����������壮