��Ŀ����
14��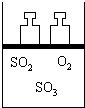 ��ͼ��ʾ����4mol SO2��2mol O2�����������ɱ�ĵ�ѹ�����У���һ���¶��·������·�Ӧ��2SO2��g��+O2��g��?2SO3��g������H��0���÷�Ӧ�ﵽƽ��״̬Aʱ��������������ʵ���Ϊ4.2mol����SO2��O2��SO3����ʼ���ʵ����ֱ���a��b��c ��ʾ���Իش�
��ͼ��ʾ����4mol SO2��2mol O2�����������ɱ�ĵ�ѹ�����У���һ���¶��·������·�Ӧ��2SO2��g��+O2��g��?2SO3��g������H��0���÷�Ӧ�ﵽƽ��״̬Aʱ��������������ʵ���Ϊ4.2mol����SO2��O2��SO3����ʼ���ʵ����ֱ���a��b��c ��ʾ���Իش���1���ﵽƽ��״̬A��������ͨ������O2����ϵ��SO2�������������С����ҪʹSO2����������ٱ�����ԭƽ��״̬A��ͬ���ɲ��õĴ�ʩ��ͨ������SO2�����ϵ���£�
��2������ʼʱa=1.2mol��b=0.6mol���Ҵﵽƽ������������������ƽ��״̬A��ͬ������ʼʱc��ȡֵΪc��0��
��3����ԭƽ��״̬A���ں��º����´ﵽ�ģ���Ҫʹ��Ӧ��ʼʱ���淽����У��Ҵﵽ��ƽ��״̬��A��ͬ������ʼʱc��ȡֵ��ΧΪ3.6��c��4��
���� ��1�����º�ѹ�£�ͨ������O2��ʹƽ�������ƶ��������SO2ת���ʣ�������ϵ��SO2�����������С����ҪʹSO2����������ٱ�����ԭƽ��״̬A��ͬ����ͨ������SO2�������ϵ����ʹƽ�������ƶ���
��2������ʼʱa=1.2mol��b=0.6mol���Ҵﵽƽ������������������ƽ��״̬A��ͬ��Ϊ��Чƽ�⣬���º�ѹ�£�����ѧ��������ȫת�����������n��SO2����n��O2��=2��1��
��3����ԭƽ��״̬A���ں��º����´ﵽ�ģ��ﵽ��ƽ��״̬��A��ͬ������ѧ��������ȫת�����������n��SO2��=4mol��n��O2��=2mol��Ҫʹ��Ӧ��ʼʱ���淽����У���cӦ����ԭƽ��ʱ�����������ʵ�������������������Ϊ0ʱ������ȷ��c�����ֵ��
��� �⣺��1���ڴﵽƽ��״̬A����������ͨ������O2��˲������������Ũ�ȣ���ʹƽ�������ƶ��������SO2ת���ʣ�������ϵ��SO2�����������С����ҪʹSO2����������ٱ�����ԭƽ��״̬A��ͬ����ͨ������SO2�������ϵ����ʹƽ�������ƶ���
�ʴ�Ϊ����С��ͨ������SO2������ϵ���£�
��2������ʼʱa=1.2mol��b=0.6mol���������ʵ���֮�ȵ�����ʼʱ�����2mol SO2��1mol O2�����ʵ���֮�ȣ�����������ת���õ���SO2��O2d�����ʵ���֮��Ҳ��2��1����c��0����ѧ��������ȫת�����������n��SO2����n��O2��=2��1��������ƽ��һ��Ϊ��Чƽ�⣬
�ʴ�Ϊ��c��0��
��3����ԭƽ���з�Ӧ������Ϊxmol����
2SO2��g��+O2��g��?2SO3��g��
��ʼ��mol����4 2 0
�仯��mol����2x x 2x
ƽ�⣨mol����4-2x 2-x 2x
��4-2x��+��2-x��+2x=4.2�����x=1.8����ԭƽ��ʱ��������Ϊ3.6mol��
��ԭƽ��״̬A���ں��º����´ﵽ�ģ��ﵽ��ƽ��״̬��A��ͬ������ѧ��������ȫת�����������n��SO2��=4mol��n��O2��=2mol��Ҫʹ��Ӧ��ʼʱ���淽����У���c��3.6��SO2��O2������Ϊ0��c��ֵ���ʱ��4molSO2��2molO2��ȫת�����Եõ�4molSO3����3.6��c��4��
�ʴ�Ϊ��3.6��c��4��
���� ���⿼�黯ѧƽ����㣬�漰��Чƽ�����⣬�ؼ����������յ�Чƽ����ɣ��Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 1mol NH5�к���5NA��N-H�� | |
| B�� | NH5��NԪ�صĻ��ϼ�Ϊ-5�� | |
| C�� | ��ˮ��Ӧʱ��ԭ����NaH��ˮ��Ӧ��ͬ | |
| D�� | ���Ҵ���Ӧʱ��NH5����ԭ |
| A�� |  װ�ÿ����ڷ��뱽���屽�Ļ���� | B�� |  װ�ÿ���������HCl��NH3���� | ||
| C�� |  ��ͼ��ʾװ�÷����Ҵ������� | D�� |  ͼ����Ͳ�з����˼ӳɷ�Ӧ |
| A�� | ú�� | B�� | Һ�� | C�� | �ȷ� | D�� | �Ҵ� |
��1������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�ӦCO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
 ��ʵ��1����v ��CO2�� ��ʾ�ķ�Ӧ����Ϊ0.13mol/��L��min���� ��ȡС����λ����ͬ����
��ʵ��1����v ��CO2�� ��ʾ�ķ�Ӧ����Ϊ0.13mol/��L��min���� ��ȡС����λ����ͬ�����ڸ÷�ӦΪ����������š����ȷ�Ӧ��ʵ��2������ƽ�ⳣ��K=$\frac{1}{6}$��
��2����֪�ڳ��³�ѹ�£�
��2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��g����H=-1275.6kJ/mol
��2CO ��g��+O2��g���T2CO2��g����H=-566.0kJ/mol
��H2O��g���TH2O��l����H=-44.0kJ/mol
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��CH3OH��l��+O2��g��=CO��g��+2H2O��l����H=-442.8 kJ/mol��
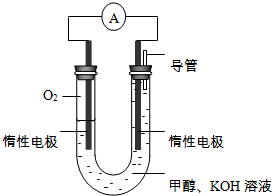
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ���������ͼ��ʾ�ĵ��װ�ã�
�ٸõ�������ĵ缫��ӦΪO2+2H2O+4e-=4OH-��
�ڹ���һ��ʱ������Һ��pH��С���õ���ܷ�Ӧ�Ļ�ѧ����ʽΪ2CH3OH+3O2+4KOH=2K2CO3+6H2O��
��4��CO2����Ȼ��ѭ��ʱ����CaCO3��Ӧ��CaCO3��һ���������ʣ���Ksp=2.8��10-9��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2��10-4mo1/L�������ɳ�������CaCl2��Һ����СŨ��Ϊ5.6��10-5mol/L��

��ش��������⣺
��д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ��S��s��+O2��g���TSO2��g����H=-297 KJ•mol-1��
�ڡ�H2=-78.64 kJ/mol��
������ͬ�����£�����1mol SO3��0.5mol O2����ﵽƽ��ʱSO3��ת����Ϊ20%����ʱ�÷�Ӧ���գ���ų��������ա���19.66 kJ��������
��2���й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005���½�40%��50%������Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ������ܵ���C������ţ���
A�����ˮ���⣺2H2O$\frac{\underline{\;���\;}}{\;}$2H2��+O2��
B������ʹˮ�ֽ����⣺2H2O��g��$\frac{\underline{\;����\;}}{\;}$2H2+O2��
C��̫������ֽ�ˮ���⣺2H2O $\frac{\underline{\;\;\;TiO_{2}\;\;\;}}{̫����}$2H2��+O2��
D����Ȼ�����⣺CH4+H2O��g�� $\stackrel{����}{?}$CO+3H2
��CO2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO2��3mol H2��һ�������·�Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ/mol�����CO2��CH3OH��g��Ũ����ʱ��仯��ͼ2��ʾ��
��3min��9min���ԣ�H2��=0.125 mol/��L•min����
����˵��������Ӧ�ﵽƽ��״̬����D�����ţ���
A����Ӧ��CO2��CH3OH�����ʵ���Ũ��֮��Ϊ1��1����ͼ�н���㣩
B�����������ܶȲ���ʱ��ı仯���仯
C��3��H-H�����ѵ�ͬʱ��4��C-H������
D��CO2����������ڻ�������б��ֲ���
��3����ҵ�ϣ�CH3OHҲ����CO��H2�ϳɣ��ο��ϳɷ�ӦCO��g��+2H2��g��?CH3OH��g����ƽ�ⳣ����
| �¶�/�� | 0 | 100 | 200 | 300 | 400 |
| ƽ�ⳣ�� | 667 | 13 | 1.9��10-2 | 2.4��10-4 | 1��10-5 |
A���ÿ��淴Ӧ������Ӧ�Ƿ��ȷ�Ӧ
B����T��ʱ��1L�ܱ������У�Ͷ��0.1molCO��0.2molH2���ﵽƽ��ʱ��CO��ת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
C����ҵ�ϲ����Ըߵ�ѹǿ��5MPa����250�棬����Ϊ�������£�ԭ������ת������ߣ�
 ��
�� ���������Ӽ��ͷǼ��Թ��ۼ��Ļ�����ĵ���ʽΪ
���������Ӽ��ͷǼ��Թ��ۼ��Ļ�����ĵ���ʽΪ ��
��