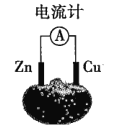
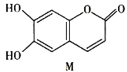
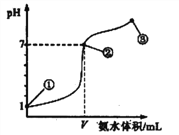
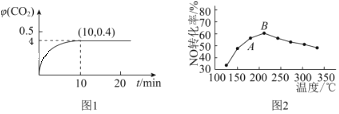
��Ŀ����
����Ŀ����ҵ���Ʊ�K2FeO4���������£�
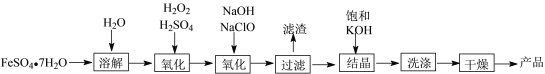
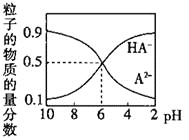
(1)����FeSO4��Һʱ����������ۺ�ϡ���ᣬ��˵�������۵�ԭ��______________________����ϡ����ԭ��_______________________��
(2)�ñ���KOH��Һ�ᾧ��ԭ����__________________��
(3)ϴ��ʱ��������ϴ�Ӽ���ԭ����_________________��
(4)���ⶨ��һ����������ʱ��ת���ʷֱ�Ϊa��b���ᾧʱ��ת����Ϊc����Ҫ�Ʊ�d Kg��K2FeO4������ҪFeSO4��7H2O___________Kg����K2FeO4����Է���������198��FeSO4��7H2O����Է���������278�����÷�����ʾ��
(5)��ⷨҲ���Ʊ�K2FeO4����KOH��Һ�����Һ�����������Խ���������FeO![]() ����д����ʱ�����ĵ缫��Ӧʽ___________________________��
����д����ʱ�����ĵ缫��Ӧʽ___________________________��
���𰸡���ֹFeSO4������ ����FeSO4��ˮ�� �ṩK+ ����K2FeO4����ʧ 139d/ 99abc Fe+8OH--6e-= FeO![]() +4H2O
+4H2O
��������
�������̿�֪����ҵ����FeSO4�Ʊ�K2FeO4�����Ļ��ϼ���+2�����ߵ�+6�ۣ��ʾ�������������Ŀ������ʵ�������ϼ۵�ת����K+����������ͨ�����뱥��KOH��Һ��FeSO4�����������ױ��������������ӣ�����ˮ�������������������������۷�ֹ������������������ˮ�⣻������ز��������ѣ�ϴ��ʱ��������ϴ�Ӽ����Լ��ٲ�Ʒ��ʧ����ҪFeSO47H2O ����������Ԫ���غ���м��㣻���ʱ����ʧ���ӣ�����������Ӧ��Fe�ڼ��������±�����ΪFeO42-���缫��ӦʽΪ��Fe+8OH--6e-=FeO42+4H2O��
(1)FeSO4��Һ�����������ױ��������������ӣ�����ˮ�������������������������۷�ֹ������������������ˮ�⣬ʵ��������FeSO4��Һʱ����������ۺ�ϡ���ᣬ�������۵�ԭ���Ƿ�ֹFeSO4����������ϡ����ԭ��������FeSO4��ˮ�⣻
(2)����������֪�ñ���KOH��Һ�ṩ�����ӽᾧ����������ؾ��壬�ñ���KOH��Һ�ᾧ��ԭ�����ṩK+���ӣ�
(3)������ز��������ѣ�ϴ��ʱ��������ϴ�Ӽ���ԭ���Ǽ��ٲ�ƷK2FeO4����ʧ��
(4)���ⶨ��һ����������ʱ��ת���ʷֱ�Ϊa��b���ᾧʱ��ת����Ϊc����Ҫ�Ʊ�d Kg��K2FeO4��Ԫ���غ�õ���Ԫ�����ʵ���![]() Kmol������Ҫ�̷�����Ϊxkg����õ�x��abc=
Kmol������Ҫ�̷�����Ϊxkg����õ�x��abc=![]() ��278�����x=
��278�����x=![]() kg=
kg=![]() kg������ҪFeSO47H2O ����Ϊ��
kg������ҪFeSO47H2O ����Ϊ��![]() ��
��![]() ��
��
(5)���ʱ����ʧ���ӣ�����������Ӧ��Fe�ڼ��������±�����ΪFeO42-���缫��ӦʽΪ��Fe+8OH--6e-=FeO42-+4H2O��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�