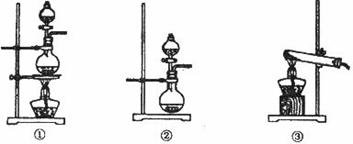
��Ŀ����
��13�֣�ij�о���ѧϰС���������ռ���������Ϣ��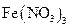 ��Һ����ʴ�������������������Ρ����Ƕ�ʴ������ԭ�����������̽����
��Һ����ʴ�������������������Ρ����Ƕ�ʴ������ԭ�����������̽����
��ʵ�顿�������������� ��Һ��Ӧ�����������ܽ⡣
��Һ��Ӧ�����������ܽ⡣
��1�������й��Ʊ��������̵�˵����ȷ���� ��
a. ����ʢ��2%��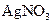 ��Һ���Թܣ��ߵ���2%�İ�ˮ��������ij���ǡ���ܽ�Ϊֹ
��Һ���Թܣ��ߵ���2%�İ�ˮ��������ij���ǡ���ܽ�Ϊֹ
b��������������Һ����2ml��ȩ��
c���Ʊ�����ʱ���þƾ��Ƶ�������Թܵײ�����
d��������Һ���н�����������
e����������Һ���ù����У���Һ��pH����
��������衿
����1�� ���������ԣ�������Ag��
���������ԣ�������Ag��
����2��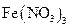 ��Һ�����ԣ��ڴ�����������
��Һ�����ԣ��ڴ����������� ������Ag��
������Ag��
�����ʵ�鷽������֤���衿
(2)��ͬѧ������ʵ����������м����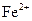 ����֤�˼���1�ij�������д��
����֤�˼���1�ij�������д�� ����Ag�����ӷ���ʽ�� ��
����Ag�����ӷ���ʽ�� ��
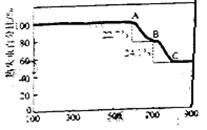
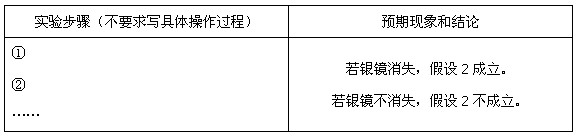
��3����ͬѧ���ʵ����֤����2�����������±������ݣ���ʾ�� �ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
�ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
��˼���뽻����
��4����ͬѧ��֤�˼���1����������ͬѧ��֤�˼���2Ҳ���������ͬѧ�ɴ˵ó����ۣ�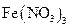 ��Һ�е�
��Һ�е� ��
�� ��������Ag�����Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ� ��
��������Ag�����Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ� ��
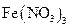 ��Һ����ʴ�������������������Ρ����Ƕ�ʴ������ԭ�����������̽����
��Һ����ʴ�������������������Ρ����Ƕ�ʴ������ԭ�����������̽������ʵ�顿��������������
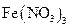 ��Һ��Ӧ�����������ܽ⡣
��Һ��Ӧ�����������ܽ⡣��1�������й��Ʊ��������̵�˵����ȷ���� ��
a. ����ʢ��2%��
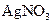 ��Һ���Թܣ��ߵ���2%�İ�ˮ��������ij���ǡ���ܽ�Ϊֹ
��Һ���Թܣ��ߵ���2%�İ�ˮ��������ij���ǡ���ܽ�Ϊֹb��������������Һ����2ml��ȩ��
c���Ʊ�����ʱ���þƾ��Ƶ�������Թܵײ�����
d��������Һ���н�����������
e����������Һ���ù����У���Һ��pH����
��������衿
����1��
 ���������ԣ�������Ag��
���������ԣ�������Ag������2��
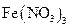 ��Һ�����ԣ��ڴ�����������
��Һ�����ԣ��ڴ����������� ������Ag��
������Ag�������ʵ�鷽������֤���衿
(2)��ͬѧ������ʵ����������м����
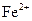 ����֤�˼���1�ij�������д��
����֤�˼���1�ij�������д�� ����Ag�����ӷ���ʽ�� ��
����Ag�����ӷ���ʽ�� ����3����ͬѧ���ʵ����֤����2�����������±������ݣ���ʾ��
 �ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
�ڲ�ͬ�����µĻ�ԭ����ϸ��ӣ���ʱ���Թ۲쵽�����������
��˼���뽻����
��4����ͬѧ��֤�˼���1����������ͬѧ��֤�˼���2Ҳ���������ͬѧ�ɴ˵ó����ۣ�
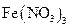 ��Һ�е�
��Һ�е� ��
�� ��������Ag�����Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ� ��
��������Ag�����Ƿ�ͬ���ͬѧ�Ľ��ۣ����������ɣ� ����1��a d e
(2)Ag+Fe3+=Ag++Fe2+
(3)
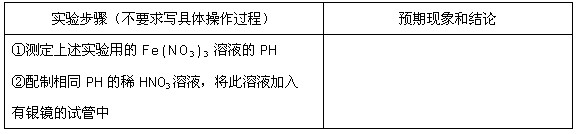
(4)��ͬ�⣻��ͬѧ�������Fe2+����ȷ��Fe3+һ��������������ͬѧ��Ȼ��֤�˴�������NO3-����������������������Һ������ʱ������û�м���NO3-�Ļ�ԭ�����˲���ȷ��NO3-�Ƿ�����������
��

��ϰ��ϵ�д�
 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
�����Ŀ
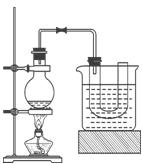
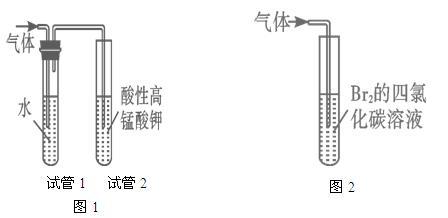
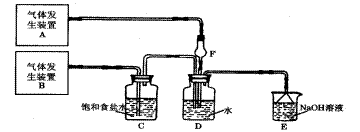
 ��
��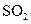 ���壬�ֲ���Na2SO3��70%��Ũ����Ϊԭ����ȡSO2������MnO2��Ũ����Ϊԭ����ȡC12���ڴ�ʵ���У�E������������__________������װ��BӦѡ����������װ���е�___________������ţ���
���壬�ֲ���Na2SO3��70%��Ũ����Ϊԭ����ȡSO2������MnO2��Ũ����Ϊԭ����ȡC12���ڴ�ʵ���У�E������������__________������װ��BӦѡ����������װ���е�___________������ţ���