题目内容
已知琥珀酸亚铁是常见治疗缺铁性贫血的补铁药剂的主要成分,该药品不溶于水但能溶于人体中的胃酸。请回答相关问题。
(1)常见治疗缺铁性贫血的补铁药剂通常都制成糖衣片,除了服用方便外它的另一个最重要的原因是 。
(2)某同学为了检测某补铁药剂药片中Fe2+的存在,设计并进行了如下实验:
a、将药片去除糖衣,碾碎。
b、将碾碎的药片粉末转移到锥形瓶中,加入试剂A,过滤,得浅绿色溶液
c、向滤液中加入硫氰化钾溶液,溶液呈淡红色,加入几滴新制氯水,溶液立即出现血红色
d、向血红色溶液中继续滴加氯水,并振荡,静止几分钟,红色褪去。请回答下列问题
①操作a中碾碎药片所使用的主要仪器是
②试剂A是 ,
③加入KSCN溶液后,在未加新制氯水的情况下,溶液中也产生了红色,其可能的原因是 。
④用方程式表示溶液出现血红色的原因 。
⑤对溶液最后褪色的原因,甲、乙两位同学首先进行了猜想:
甲同学认为:可能是溶液中的+3价Fe又被还原为+2价Fe
乙同学认为:可能是溶液中的SCN-被过量的氯水氧化
基于乙同学的猜想,请设计实验方案,验证乙同学的猜想是否正确。写出有关的实验操作、预期现象和结论。
(1)常见治疗缺铁性贫血的补铁药剂通常都制成糖衣片,除了服用方便外它的另一个最重要的原因是 。
(2)某同学为了检测某补铁药剂药片中Fe2+的存在,设计并进行了如下实验:
a、将药片去除糖衣,碾碎。
b、将碾碎的药片粉末转移到锥形瓶中,加入试剂A,过滤,得浅绿色溶液
c、向滤液中加入硫氰化钾溶液,溶液呈淡红色,加入几滴新制氯水,溶液立即出现血红色
d、向血红色溶液中继续滴加氯水,并振荡,静止几分钟,红色褪去。请回答下列问题
①操作a中碾碎药片所使用的主要仪器是
②试剂A是 ,
③加入KSCN溶液后,在未加新制氯水的情况下,溶液中也产生了红色,其可能的原因是 。
④用方程式表示溶液出现血红色的原因 。
⑤对溶液最后褪色的原因,甲、乙两位同学首先进行了猜想:
甲同学认为:可能是溶液中的+3价Fe又被还原为+2价Fe
乙同学认为:可能是溶液中的SCN-被过量的氯水氧化
基于乙同学的猜想,请设计实验方案,验证乙同学的猜想是否正确。写出有关的实验操作、预期现象和结论。
(1)防止二价铁被氧化为三价铁而变质(1分)
(2)①研钵(1分)
②稀盐酸(或稀硫酸溶液)(1分)
③少量的Fe2+被空气中的氧气氧化(2分)。
④2Fe2++Cl2=2Fe3++2Cl-(1分) ;Fe3++SCN-=[Fe(SCN)]2+ (或Fe3++3SCN-=Fe(SCN) 3)(1分)
⑤取少量褪色后溶液,加入KSCN溶液;如果溶液变红色,说明乙同学的猜想是合理的;如果溶液不变红色,说明乙同学的猜想是不合理的(2分)。
或取少量褪色后溶液,加入FeCl3溶液;如果溶液仍不变红色,说明乙同学的猜想是合理的;如果溶液变红色,说明乙同学的猜想是不合理的。
(2)①研钵(1分)
②稀盐酸(或稀硫酸溶液)(1分)
③少量的Fe2+被空气中的氧气氧化(2分)。
④2Fe2++Cl2=2Fe3++2Cl-(1分) ;Fe3++SCN-=[Fe(SCN)]2+ (或Fe3++3SCN-=Fe(SCN) 3)(1分)
⑤取少量褪色后溶液,加入KSCN溶液;如果溶液变红色,说明乙同学的猜想是合理的;如果溶液不变红色,说明乙同学的猜想是不合理的(2分)。
或取少量褪色后溶液,加入FeCl3溶液;如果溶液仍不变红色,说明乙同学的猜想是合理的;如果溶液变红色,说明乙同学的猜想是不合理的。
二价铁极易被空气中氧气等氧化剂氧化为三价铁而变质,故补铁药剂都用糖衣包裹;溶液最后退色的原因要么是没有了Fe3+,要么是没有了SCN-,故可向已退色的溶液中继续加入这两种离子中的任意一种来检验。

练习册系列答案
相关题目
 究反应物浓度、温度、催化剂对反应速率的影响,通过变换这些实验条件,至少需要完成_______个实验进行对比即可得出结论。
究反应物浓度、温度、催化剂对反应速率的影响,通过变换这些实验条件,至少需要完成_______个实验进行对比即可得出结论。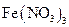 溶液可以蚀刻银,制作美丽的银饰。他们对蚀刻银的原因进行了如下探究:
溶液可以蚀刻银,制作美丽的银饰。他们对蚀刻银的原因进行了如下探究: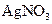 溶液的试管,边滴入2%的氨水,至最初的沉淀恰好溶解为止
溶液的试管,边滴入2%的氨水,至最初的沉淀恰好溶解为止 具有氧化性,能氧化Ag。
具有氧化性,能氧化Ag。 能氧化Ag。
能氧化Ag。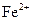 ,验证了假设1的成立。请写出
,验证了假设1的成立。请写出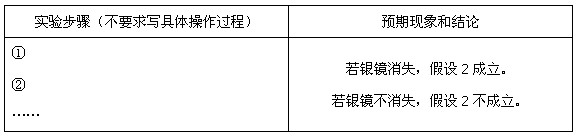

 4NO2(g);△H>0,下表为反应在某温度下的部分实验数据
4NO2(g);△H>0,下表为反应在某温度下的部分实验数据