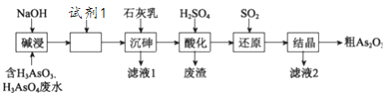
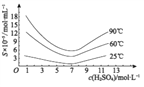
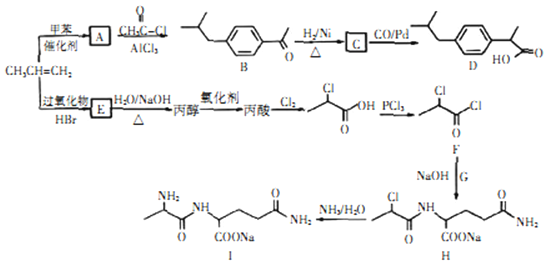
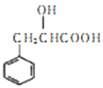
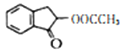
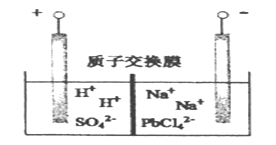
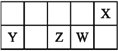
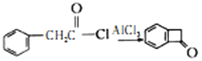��Ŀ����
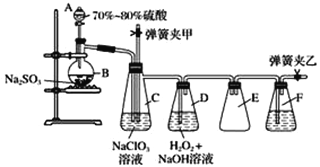
����Ŀ��ij��Һ�п��ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Fe2+��Al3+��Na+��ijͬѧΪ��ȷ����ɷ֣�ȡ������Һ����Ʋ����������ʵ�飺
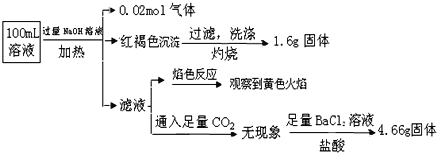
����˵����ȷ����
A. ԭ��Һ��c(Fe3+)=0.2mol/L
B. ��Һ��������4�����Ӵ��ڣ�����Cl-һ������
C. SO42-��NH4+��Na+һ�����ڣ�CO32-һ��������
D. Ҫȷ��ԭ��Һ���Ƿ���Fe2+�������Ϊ:ȡ����ԭ��Һ���Թ��У�����������ˮ���������ټ�KSCN��Һ����Һ��Ѫ��ɫ������Fe2+
���𰸡�B
����������Һ�м��������NaOH��Һ�������ȣ��������壬������ӦΪNH3����ԭ��Һ�к���NH4����n(NH4��)=0.02mol���к��ɫ����������˵��ԭ��Һ��Fe2����Fe3��������һ�֣�1.6g����ΪFe2O3��ԭ��Һ�к�����ԭ�ӵ����ʵ���Ϊ1.6��2/160mol=0.02mol���������ӹ��棬ԭ��Һ��һ��������CO32������Һ����ɫ��Ӧ���۲쵽��ɫ���棬����˵��ԭ��Һ�к���Na������Ϊ�������������ǹ��������������к�����Ԫ�أ���Һ��ͨ��������CO2��������˵��ԭ��Һ�в���Al3��������������BaCl2��Һ�����ᣬ�õ�������˵��ԭ��Һ�к���SO42����n(SO42��)=4.66/233mol=0.02mol������ԭ��Һ��ֻ��Fe2�������ݵ���غ㣬����������������ʵ���(0.02��0.02��2)mol=0.06mol������������������ʵ���0.04mol��˵��ԭ��Һ��һ������Cl����ͬ���������Fe3������ԭ��Һ��һ������Cl����A�������������������жϣ��Ƿ���Fe2������A����B������������������B��ȷ��C������������������C����D����Ϊԭ��Һ�п��ܺ���Fe3��������KSCN��Һ����ʵ��������ţ�Ӧ�ø��������Һ�����Ϻ�ɫ��ȥ��˵������Fe2������D����