��Ŀ����
1�� �й�Ůҩѧ�����������������ض�ű�����������ö���Ϊ2015��ŵ��������ҽѧ�������֮һ������˵������ȷ���ǣ�������
�й�Ůҩѧ�����������������ض�ű�����������ö���Ϊ2015��ŵ��������ҽѧ�������֮һ������˵������ȷ���ǣ�������| A�� | ����������ȡ�����صķ���������ȡԭ��Ϊ��������ȡ��һ�ֻ�ѧ�仯 | |
| B�� | �����صķ���ʽΪC15H22O5���������л��� | |
| C�� | �˹��ϳ������ؾ����˳��ڵ�ʵ���о���ʵ���ǻ�ѧ�о�����Ҫ�ֶ� | |
| D�� | �ִ���ѧ���������У�����Ԫ�ط�����ȷ���������е�C��H��OԪ�� |
���� A����ȡ�������ʵ��ܽ��Խ��з��룻
B�������ش��л����з������������ʽΪC15H22O5��
C����ѧʵ���ȷ���л������ɡ��ṹ�����ʵȣ�
D��Ԫ�ط����ǿ�ȷ��Ԫ�����࣮
��� �⣺A����ȡ�������ʵ��ܽ��Խ��з��룬Ϊ�����仯����A����
B�������ش��л����з������������ʽΪC15H22O5�������л����B��ȷ
C����ѧʵ���ȷ���л������ɡ��ṹ�����ʵȣ�Ϊ��ѧ�о�����Ҫ�ֶΣ���C��ȷ��
D��Ԫ�ط����ǿ�ȷ��Ԫ�����࣬��D��ȷ��
��ѡA��
���� ���⿼���л���Ľṹ�����ʣ�Ϊ��Ƶ���㣬�����ڻ�ѧ����������Ŀ��飬����������ѧ�����õĿ�ѧ���������ѧϰ�Ļ����ԣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
11�����з�Ӧ������Һ��һ��ֻ��һ�����ʵ��ǣ�������
| A�� | ��AlCl3��Һ�е���NaOH��Һ | |
| B�� | ��NaOH��Һ��ͨ��SO2���� | |
| C�� | ��Ũ�����м������ͭ�� | |
| D�� | ��MgCl2��H2SO4�Ļ��Һ�е������Ba��OH��2��Һ |
9��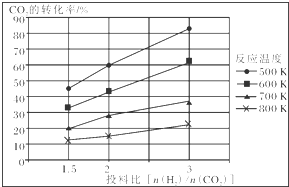 ȼú�����еĵ������NOx����������̼�����壬�������з�����������ʵ�ֽ��ܼ��š��������õȣ�
ȼú�����еĵ������NOx����������̼�����壬�������з�����������ʵ�ֽ��ܼ��š��������õȣ�
��1����ȼú����������������ʱ�������ü������ԭ�������
��CH4 ��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-570kJ•mol-1
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ•mol-1
��CH4 ��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H=-865 kJ•mol-1��
��2����ȼú�����е�CO2ת��Ϊ���ѵķ�Ӧԭ��Ϊ��2CO2��g��+6H2��g��$\stackrel{����}{?}$CH3OCH3��g��+3H2O��g��
��֪��ѹǿΪa MPa�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת���ʼ���ͼ��
�ٴ˷�ӦΪ���ȣ�����ȡ��������ȡ��������¶Ȳ��䣬���Ͷ�ϱ�[$\frac{n��{H}_{2}��}{n��C{O}_{2}��}$]����K�����䣨���������С�����䡱����
����a MPa��һ���¶��£���6mol H2��2mol CO2��2L�ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������CH3OCH3���������ԼΪ16.7%����$\frac{1}{6}$������ʱCO2��ת����Ϊ80%
��3������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ
CO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
��650��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ����ֵΪ$\frac{8}{3}$���÷�Ӧ���淴ӦΪ�ţ�������š����ȷ�Ӧ
����ʵ��3Ҫ�ﵽ��ʵ��2��ͬ��ƽ��״̬���������ʵ����������ֱ���ȣ�����t��3min����a��bӦ����Ĺ�ϵ��b=2a��a��1���ú�a��b����ѧʽ��ʾ����
 ȼú�����еĵ������NOx����������̼�����壬�������з�����������ʵ�ֽ��ܼ��š��������õȣ�
ȼú�����еĵ������NOx����������̼�����壬�������з�����������ʵ�ֽ��ܼ��š��������õȣ���1����ȼú����������������ʱ�������ü������ԭ�������
��CH4 ��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-570kJ•mol-1
��CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ•mol-1
��CH4 ��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H=-865 kJ•mol-1��
��2����ȼú�����е�CO2ת��Ϊ���ѵķ�Ӧԭ��Ϊ��2CO2��g��+6H2��g��$\stackrel{����}{?}$CH3OCH3��g��+3H2O��g��
��֪��ѹǿΪa MPa�£��÷�Ӧ�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ת���ʼ���ͼ��
�ٴ˷�ӦΪ���ȣ�����ȡ��������ȡ��������¶Ȳ��䣬���Ͷ�ϱ�[$\frac{n��{H}_{2}��}{n��C{O}_{2}��}$]����K�����䣨���������С�����䡱����
����a MPa��һ���¶��£���6mol H2��2mol CO2��2L�ܱ������л�ϣ����÷�Ӧ�ﵽƽ��ʱ�����ƽ��������CH3OCH3���������ԼΪ16.7%����$\frac{1}{6}$������ʱCO2��ת����Ϊ80%
��3������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У����з�Ӧ
CO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
| ʵ �� �� | �� �� /�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO | |||
| 1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
| 3 | 900 | a | b | c | d | t |
����ʵ��3Ҫ�ﵽ��ʵ��2��ͬ��ƽ��״̬���������ʵ����������ֱ���ȣ�����t��3min����a��bӦ����Ĺ�ϵ��b=2a��a��1���ú�a��b����ѧʽ��ʾ����
16�����з�Ӧ�����ӷ���ʽ��ȷ���ǣ�������
| A�� | ̼�������ϡ�����У�CaCO3+2H+�TCa2++H2O+CO2�� | |
| B�� | ����ϡ���ᷴӦ��2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ̼��������Һ�е���NaOH��Һ��HCO${\;}_{3}^{-}$+OH-�TH2O+CO2�� | |
| D�� | ������Һ�м�������������Һ��Ba2++SO${\;}_{4}^{2-}$�TBaSO4�� |
6��������Һ��Cl-Ũ�������ǣ�������
| A�� | 200mL 2 mol•L-1 MgCl2��Һ | B�� | 1000mL 2.5 mol•L-1 NaCl��Һ | ||
| C�� | 300mL 5 mol•L-1 KCl��Һ | D�� | 250mL 1 mol•L-1 AlCl3��Һ |
10��ά����ͨ���������ǵ��ܽ�������з��࣬���ǻ����ܽ���ˮ�л����ܽ���֬���У�����ά����������ˮ����ά���ص��ǣ�������
| A�� | ����A | B�� | ����C�� | C�� | ����D | D�� | ����E |
11�������йط�Ӧ����ʽ����д��ȷ���ǣ�������
| A�� | ��ĭ����������ԭ���漰�����ӷ�Ӧ����ʽΪ��Al3++3HCO3-=Al��OH��3��+3CO2�� | |
| B�� | ��Na2S��Һ����FeCl3��Һ�е����ӷ�Ӧ����ʽΪ��3S2-+2Fe3++6H2O=2Fe��OH��3��+3H2S�� | |
| C�� | ��֪H2��ȼ����Ϊ283 kJ•mol-1�����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ��2 H2 ��g��+O2��g��=2 H2O��l����H=-566 kJ•mol-1 | |
| D�� | ��1L 1 mol/L NaOH��Һ�м���һ����ŨH2SO4��Һ��ǡ����ȫ��Ӧʱ�����ų�65 kJ�����������ʾ�÷�Ӧ���к��ȵ��Ȼ�ѧ����ʽΪ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-65kJ•mol-1 |