��Ŀ����
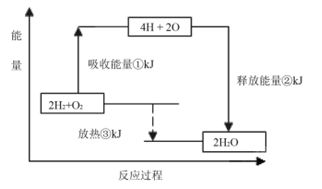
��֪�ڷ�������ʱ������(N2H4)Ϊȼ�Ϻ�NO2�������������߷�Ӧ����N2��ˮ������
��֪��N2(g)��2O2(g)===2NO2(g) �� ��H����67.7kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H����534kJ��mol��1
������NO2��Ӧ���Ȼ�ѧ����ʽΪ�� ��
��֪��N2(g)��2O2(g)===2NO2(g) �� ��H����67.7kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H����534kJ��mol��1
������NO2��Ӧ���Ȼ�ѧ����ʽΪ�� ��
| A��N2H4(g)��NO2(g)===3/2N2(g)��2H2O(g) ��H����567.85kJ��mol��1 |
| B��N2H4(g)��NO2(g)===3/2N2(g)��2H2O(g) ��H����567.85kJ��mol��1 |
| C��N2H4(g)��NO2(g)===3/2N2(g)��2H2O(l) ��H����567.85kJ��mol��1 |
| D��N2H4(g)��NO2(g)===3/2N2(g)��2H2O(l) ��H����567.85kJ��mol��1 |
B
���ݸ�˹���ɿ�֪���ڣ��١�2���õ�N2H4(g)��NO2(g)===3/2N2(g)��2H2O(g)�����Ը÷�Ӧ�ķ�Ӧ�Ȧ�H����534kJ��mol��1��67.7kJ��mol��1��2����567.85kJ��mol��1����ѡB��

��ϰ��ϵ�д�
�����Ŀ

 ��
�� 

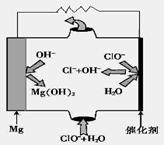
 ��
��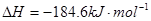 ����Ӧ
����Ӧ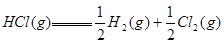 �ġ�HΪ���� ��
�ġ�HΪ���� ��