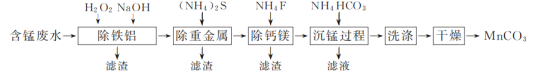
��Ŀ����
����Ŀ����15�֣���̼���ʵļ�ֵ��ת���������ڡ���̼���Ϳɳ����Է�չ��������Ҫ���о���ֵ����ش��������⣺
��1����֪CO�����л�ѧ��ΪC��O����صĻ�ѧ�������������£�
��ѧ�� | H��O | C��O | C=O | H��H |
E/(kJ��mol1) | 463 | 1075 | 803 | 436 |
CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H=___________kJ��mol1���������������COƽ��ת���ʵĴ�ʩ��_______________�����ţ���
CO2(g)��H2(g) ��H=___________kJ��mol1���������������COƽ��ת���ʵĴ�ʩ��_______________�����ţ���
a������ѹǿ b�������¶�
c�����ԭ������H2O�ı��� d��ʹ�ø�Ч����
��2���ö��Ե缫���KHCO3��Һ���ɽ������е�CO2ת��Ϊ�����(HCOO)��Ȼ���һ�������Ƶ���Ҫ�л�����ԭ�ϼ��ᡣCO2������Ӧ�ĵ缫��ӦʽΪ________________������������ת��1 mol���ӣ���������������������״����Ϊ_________L��
��3���ұ���������ȡ����ϩ�ķ�ӦΪ��![]() (g)��CO2(g)
(g)��CO2(g)![]()
![]() (g)��CO(g)��H2O(g)���䷴Ӧ�������£�
(g)��CO(g)��H2O(g)���䷴Ӧ�������£�
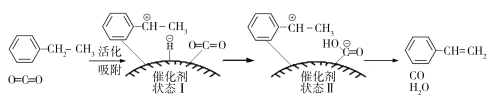
����ԭ�ϵ�״̬��____________��������ų��������ա�����
��һ���¶��£�������ܱ������г���2 mol�ұ���2 mol CO2����ʼѹǿΪp0��ƽ��ʱ���������������ʵ���Ϊ5 mol���ұ���ת����Ϊ_______����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp=_______��[�����ѹ(p��)=������ѹ(p��)�������������]
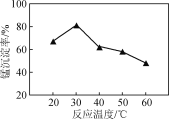
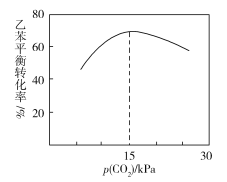
���ұ�ƽ��ת������p(CO2)�Ĺ�ϵ����ͼ��ʾ��������ұ�ƽ��ת��������p(CO2)�仯���仯��ԭ��________________________________________________��
���𰸡�41 bc 2CO2��2e��H2O===HCOO��![]() ��CO2��2e��H2O===HCOO��OH���������ɣ� 5.6 ���� 50% 0.25p0 ����CO2ѹǿ����CO2Ũ�������ұ�ƽ��ת��������CO2ѹǿ����������ɴ��������ұ����������½�
��CO2��2e��H2O===HCOO��OH���������ɣ� 5.6 ���� 50% 0.25p0 ����CO2ѹǿ����CO2Ũ�������ұ�ƽ��ת��������CO2ѹǿ����������ɴ��������ұ����������½�
��������
��1����H=463 kJ��mol1��2��1075 kJ��mol1803 kJ��mol1��2436 kJ��mol1=41 kJ��mol1��
��2��CO2ת��ΪHCOO�õ�2�����ӣ���OHƽ���ɣ��缫��ӦʽΪCO2��2e��H2O===HCOO��OH��2CO2��2e��H2O===HCOO��![]() ���������������������������ת��1 mol���ӣ������������������״����Ϊ5.6 L��
���������������������������ת��1 mol���ӣ������������������״����Ϊ5.6 L��
��3������ԭ�ϵ�״̬������ѧ���Ķ��ѣ���Ҫ����������
�����ұ���Ӧ��x mol��
![]() (g)��CO2(g)
(g)��CO2(g)![]()
![]() (g)��CO(g)��H2O(g)
(g)��CO(g)��H2O(g)
n0/mol 2 2 0 0 0
��n0/mol x x x x x
[n]/mol 2x 2x x x x
��ã�4��x=5
x=1
�ұ���ת����Ϊ![]() ��100%=50%
��100%=50%
ƽ���ѹǿΪ![]() ��p0=1.25 p0��Kp=
��p0=1.25 p0��Kp=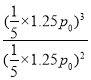 =0.25 p0
=0.25 p0
��һ����Χ�ڣ�p(CO2)Խ��˵����ԭ����CO2�����Խ�ߣ����ұ�ƽ��ת����Խ�ߣ��������������ڴ��������ϣ���CO2�ڴ������������ʹ���ʱ��������ұ��ڴ���������������½���ʹ�ұ�ƽ��ת��������p(CO2)������С��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�