��Ŀ����
����Ŀ����.CO2�������Ƽ״��Ǽ���ǰ������Դ���о�������Ҫ��Ӧ�У�
i.CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=-49.4kJ��mol-1
CH3OH(g)+H2O(g) ��H1=-49.4kJ��mol-1
ii.CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2=+41.2kJ��mol-1
CO(g)+H2O(g) ��H2=+41.2kJ��mol-1
iii.CO(g)+2H2(g)![]() CH3OH(g) ��H3
CH3OH(g) ��H3
��1����H3=___kJ��mol-1��
��2����֪��Ӧi�����ʷ���Ϊv��=k��x(CO2)��x3(H2)��v��=k����x(CH3OH)��x(H2O)��k����k����Ϊ���ʳ�����ֻ���¶��йأ�xΪ���ʵ��������������ʵ�������ƽ�ⳣ��Kx=___(��k����k����ʾ)��
��3��5MPaʱ����ij�ܱ������а�Ͷ�ϱ�n(H2)��n(CO2)=3��1����H2��CO2����Ӧ��ƽ��ʱ����ø���ֵ����ʵ����������¶ȱ仯��������ͼ��ʾ��
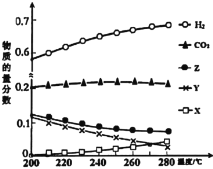
��ͼ��Y����___(�ѧʽ)��
��250��ʱ��Ӧii��Kx___1(��������������������=��)
�����д�ʩ�У�һ������״����ʵ���___��
A.��������CO B.ʹ�ô���
C.ѭ������ԭ���� D.�����¶�
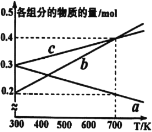
��4����10MPa�½�H2��CO��һ������Ͷ�ϣ�ƽ��״̬ʱ����ֵ����ʵ������¶ȵĹ�ϵ��ͼ��ʾ������b����������Ϊ____(�ѧʽ)���¶�Ϊ700Kʱ���÷�Ӧ��ƽ�ⳣ��KP=___(MPa)-2(����÷�����ʾ)��
��.�ں��������У�ʹ��ij�ִ����Է�ӦNO2(g)+SO2(g)![]() SO3(g)+NO(g) ��H<0
SO3(g)+NO(g) ��H<0
�������ʵ��̽�����ı�Ͷ�ϱ�[n(SO2)��n(NO2)]���ж���ʵ��(����ʵ����¶ȿ�����ͬ��Ҳ���ܲ�ͬ)���ⶨSO2��ƽ��ת����[��(SO2)]ʵ������ͼ��ʾ��
��֪��KR=16��KZ=![]() ��
��
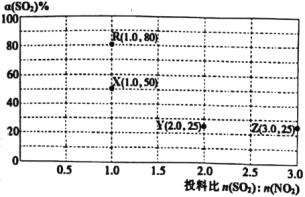
��5�����Ҫ��ͼ��R���ƽ��״̬�ı�ΪX���ƽ��״̬��Ӧ��ȡ�Ĵ�ʩ��___��
��6��ͨ������ȷ��ͼ��R��X��Y��Z�ĵ����¶���ȵĵ���__��
���𰸡���90.6 ![]() CH3OH < B��D H2
CH3OH < B��D H2 ![]() �����¶� X��Z
�����¶� X��Z
��������
��.��1�����ݸ�˹���ɼ�����H3��
��2��Kx=![]() �����ݷ�Ӧ�ﵽƽ��ʱv��= v���������ʵ�������ƽ�ⳣ��Kx��
�����ݷ�Ӧ�ﵽƽ��ʱv��= v���������ʵ�������ƽ�ⳣ��Kx��
��3�������¶ȣ���Ӧi�����ƶ�����Ӧii�����ƶ�����Ӧiii�����ƶ���
��4�������¶ȣ���Ӧiii�����ƶ���H2��CO��2:1�ı�������CH3OH�����ʵ�����С��
��. ��5��ͼ��R���ƽ��״̬�ı�ΪX���ƽ��״̬����(SO2)��С��ƽ�������ƶ���
��6��ƽ�ⳣ����ͬ���¶���ͬ��
��.��1��i.CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1=-49.4kJ��mol-1
CH3OH(g)+H2O(g) ��H1=-49.4kJ��mol-1
ii.CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2=+41.2kJ��mol-1
CO(g)+H2O(g) ��H2=+41.2kJ��mol-1
���ݸ�˹���� i- ii��CO(g)+2H2(g)![]() CH3OH(g) ��H3= -49.4kJ��mol-1 -41.2kJ��mol-1=��90.6 kJ��mol-1��
CH3OH(g) ��H3= -49.4kJ��mol-1 -41.2kJ��mol-1=��90.6 kJ��mol-1��
��2��Kx=![]() ����Ӧ�ﵽƽ��ʱv��= v������k��x(CO2)��x3(H2) =k����x(CH3OH)��x(H2O)��
����Ӧ�ﵽƽ��ʱv��= v������k��x(CO2)��x3(H2) =k����x(CH3OH)��x(H2O)��![]() =
=![]() ������Kx =
������Kx =![]() ��
��
��3���������¶ȣ���Ӧi�����ƶ�����Ӧiii�����ƶ����Լ״��ĺ������Լ�С����Ӧii�����ƶ���CO��������ˮ�ĺ����仯С�ڼ״�����ͼ��Y����CH3OH��X����CO��Z����H2O��
��250��ʱ��Ӧii��Kx=![]() ������ͼʾCO ��H2O�����ʵ�������С��CO2 ��H2������Kx<1��
������ͼʾCO ��H2O�����ʵ�������С��CO2 ��H2������Kx<1��
��A.��������CO ����Ӧiii�����ƶ�������״��IJ��ʣ��ʲ�ѡA��
B.ʹ�ô�����ƽ�ⲻ�ƶ���һ��������״��IJ��ʣ���ѡB��
C.ѭ������ԭ���������ԭ�������ʣ�����״��IJ��ʣ��ʲ�ѡC��
D.�����¶ȣ���Ӧi����Ӧiii�������ƶ���һ��������״��IJ��ʣ���ѡD��
��4�������¶ȣ���Ӧiii�����ƶ���H2��CO��2:1�ı�������CH3OH�����ʵ�����С����ͼ��a����CH3OH��c����CO��b����H2��
�¶�Ϊ700Kʱ���÷�Ӧ��ƽ�ⳣ��KP=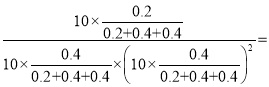
![]() (MPa)-2��
(MPa)-2��
��. ��5��ͼ��R���ƽ��״̬�ı�ΪX���ƽ��״̬����(SO2)��С��ƽ�������ƶ������Ըı�����������������¶ȣ�
��6��X�㣺
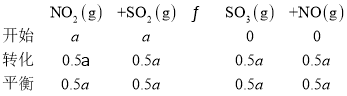
KX=![]() ��
��
Y�㣺

Ky=![]() ��
��
KR=16��KZ=l��
����ͼ��R��X��Y��Z�ĵ����¶���ȵĵ���X��Z��

����Ŀ��2019��ŵ������ѧ��������λ��������ӵ�صĿ�ѧ�ҡ�TiS2��LiCoO2��LiFePO4��LiMnO2��Cu���Ļ�����ȶ����о���صij��ò��ϡ���ش��������⡣
��1��Co4+�д���__�ֲ�ͬ�����ĵ��ӡ�
��2����Ԥ���һ�����ܣ�Cu__Zn(����������������)����˵��������ɣ�__��
��3����֪���л�������۵㣺
������ | AlF3 | GaF3 | AlCl3 |
�۵�/�� | 1040 | 1000 | 194 |
������±������۵���������ԭ���ǣ�___��
��4��ֱ���������ε��������и��ӵĽṹ������������ӡ�����������ӽṹ��ͼ��

������������ӵĻ�ѧʽ����ͨʽ��ʾΪ___(��n����Pԭ����)��
��5�����������ṹ��ͼ1��ʾ��������������4�����ͺ�4������С�����幹����ͼ2������������Ļ�ѧʽΪ___���ھ����У�ijЩԭ��λ������ԭ��Χ�ɵĿ�϶�У���ͼ3����ԭ�Ӿ�λ�������4��ԭ��Χ�ɵ����������϶�С������������У�Al3+λ��O2-�γɵ�___��϶�С��������ӵ�������NA��ʾ��������������ܶ�Ϊ___g��cm-3(�м���ʽ���ɣ����ػ���)��