��Ŀ����
��12�֣���������̼�ܵ����Ƴɹ������˿Ƽ��Ľ������õ绡���ϳɵ�����̼�ܳ����д���������̼������������̼���������������������ᴿ����Ӧ�еķ�Ӧ�����������C��CO2��H2SO4��KCr2O7��Cr2��SO4��2��H2O�������ʡ�
��1������̼��������ɢ��һ���ܼ��У��γ��ȶ��ķ�ɢϵ�����ߵ�������
�ٶ����ЧӦ�ڼ��뱥�ͣ�NH4��2SO4��Һ�����۳��ۿ�ͨ����Ĥ
��2����������������գ���ƽ��ѧ����ʽ�����������ת�Ƶķ������Ŀ��
C+ + H2SO4�� K2SO4+ + Cr2��SO4��3+ H2O
��3������״����4��48 L����������ͨ��������NaOH��Һ�г�ַ�Ӧ����Һ�������ε�����Ϊ19��0g��
��I����Ҫʹ���ɵ��ε�������Ϊ25��2 g����Ӧ��������Һ��ͨ������� g��
���������ɵ�19��0 g������Һ�м���һ����ij���ʣ���ַ�Ӧ��ѹ���������õ�������21��2 g Na2CO3���塣��
����ֻ�ܼ���0��05 molij���ʣ����������ʿ����� �� ��
����ֻ�ܼ���0��10 molij���ʣ����������ʿ����� �� ��
��1������̼��������ɢ��һ���ܼ��У��γ��ȶ��ķ�ɢϵ�����ߵ�������
�ٶ����ЧӦ�ڼ��뱥�ͣ�NH4��2SO4��Һ�����۳��ۿ�ͨ����Ĥ
��2����������������գ���ƽ��ѧ����ʽ�����������ת�Ƶķ������Ŀ��
C+ + H2SO4�� K2SO4+ + Cr2��SO4��3+ H2O
��3������״����4��48 L����������ͨ��������NaOH��Һ�г�ַ�Ӧ����Һ�������ε�����Ϊ19��0g��
��I����Ҫʹ���ɵ��ε�������Ϊ25��2 g����Ӧ��������Һ��ͨ������� g��
���������ɵ�19��0 g������Һ�м���һ����ij���ʣ���ַ�Ӧ��ѹ���������õ�������21��2 g Na2CO3���塣��
����ֻ�ܼ���0��05 molij���ʣ����������ʿ����� �� ��
����ֻ�ܼ���0��10 molij���ʣ����������ʿ����� �� ��

��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

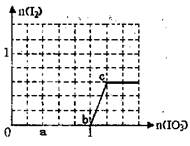
 ��
�� bO2��Cr3����Ӧ��������Cr2O72����Pb2����������1mol C
bO2��Cr3����Ӧ��������Cr2O72����Pb2����������1mol C r2O72��
r2O72��
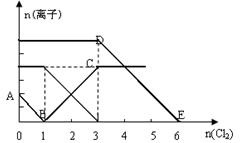
 e2+)���仯���
e2+)���仯���