��Ŀ����
12�� ���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�ã����������Ʊ��ߴ��ȵ����ᣨ����ṹ��ʽ��ͼ1���������������dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȣ����������գ�
���ʼ��仯�����ڹ�ҵ���������Ź㷺��Ӧ�ã����������Ʊ��ߴ��ȵ����ᣨ����ṹ��ʽ��ͼ1���������������dz��õ�ˮ���������������ƣ�NaH2PO2�������ڻ�ѧ�����ȵȣ����������գ���1����Ԫ��ԭ�Ӻ������������Ų�ʽΪ3S23P3��NaH2PO2���漰������Ԫ�أ����ǵ�ԭ�Ӱ뾶��С�����˳��ΪH��O��P��Na����Ԫ�ط��ţ���
��2����ԭ�Ӻ�����3�ֲ�ͬ�����ĵ��ӣ�
��3������Ʒ���Ӽ��������������Na+���������������ɵģ�ij�ֶ��������Ľṹ��ͼ2��
����ԭ�ӵ��ӻ�����Ϊsp3�������ֶ�������ƵĻ�ѧʽΪNan+2PnO3n+1
��4���������ƣ�NaH2PO2�������ڻ�ѧ��������ѧ��������Һ�к���Ni2+��H2PO2-����һ���������ܷ������·�Ӧ����Ni��H2PO3-��д��������Ӧ���ӷ���ʽH2O+Ni2++H2PO2-�TNi+H2PO3-+2H+��
���� ��1��������ԭ�Ӻ�������Ų�д�����������Ų�ʽ��NaH2PO2���漰������Ԫ��ΪNa��H��P��O��ԭ�ӵĵ��Ӳ���Խ�࣬ԭ�Ӱ뾶Խ�������Ӳ���ͬ���˵����Խ��ԭ�Ӱ뾶ԽС��
��2��������ԭ�Ӻ��⺬�е��ܼ��������жϣ�
��3���ٸ��ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
���ɸ����Ķ��������ṹʽ֪������n�������������ӣ��൱����n�������������ȥ���ˣ�n-1����ԭ�ӣ��Ӷ�ȷ������ʽ��
��4������������ԭ��Ӧ�л��ϼ۱仯�������غ㶨���жϷ�Ӧ������������ƽ��Ӧ����ʽ���жϻ�ԭ���
��� �⣺��1����Ԫ��ԭ�Ӻ��������5�����ӣ����������Ų�ʽΪ��3S23P3��NaH2PO2�к��е�Ԫ���У�Hԭ�ӵ��Ӳ����٣�ԭ�Ӱ뾶��С���������ԭ�ӵ�ԭ�Ӱ뾶����ԭ�Ӻ���ԭ�Ӷ������������Ӳ㣬��ԭ�ӵĺ˵����������ԭ�ӣ�������ԭ�ӵ�ԭ�Ӱ뾶С����ԭ�ӣ��������ǵ�ԭ�Ӱ뾶��ϵΪH��O��P��Na��
�ʴ�Ϊ��3S23P3�� H��O��P��Na��
��2������1s 2s 2p 3���ܼ���������ԭ�ӹ���3��������ͬ�ĵ��ӣ��ʴ�Ϊ��3��
��3������ԭ���γ����ĸ��Ҽ���û�йµ��Ӷԣ��۵��Ӷ���Ϊ4���ʳ�sp3�ӻ����ʴ�Ϊ��sp3��
���ɸ����Ķ��������ṹʽ֪������n�������������ӣ��൱����n�������������ȥ���ˣ�n-1����ԭ�ӣ��������Ϊ-2����3n+1��+5n=-��n+2�����ɻ��ϼ۹���֪�����Ƶ����ΪNan+2PnO3n+1���ʴ�Ϊ��Nan+2PnO3n+1��
��4����������ԭ��Ӧ�л��ϼ�������Ƚ�����ƽ����Ԫ�صĻ��ϼ۽�����2�ۣ���Ԫ�صĻ��ϼ����ߵ�2�ۣ����Ը���ԭ���غ��ϵ���غ�ɵ���ƽ�ķ���ʽΪ��H2O+Ni2++H2PO2-�TNi+H2PO3-+2H+���ʴ�Ϊ��H2O+Ni2++H2PO2-�TNi+H2PO3-+2H+��
���� ���⿼����������ԭ��Ӧ����ƽ�����ӱȽϴ�С�Ƚϡ�ͬ����Ԫ�����ʵݱ������ԭ�ӽṹ�Ĺ�ϵ����Ŀ�Ѷ��еȣ������漰�����ݽ϶࣬�����ϴ�ֿ�����ѧ����ѧ��֪ʶ�����������

| A�� | $\frac{{c��O{H^-}��}}{{c��N{H_3}•{H_2}O��}}$ | B�� | $\frac{{c��N{H_3}•{H_2}O��}}{{c��O{H^-}��}}$ | ||
| C�� | c��H+����c��OH--���ij˻� | D�� | OH-�����ʵ��� |
| A�� | �Ȼ���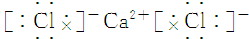 | B�� | ����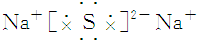 | ||
| C�� | ������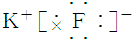 | D�� | ��������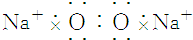 |
| A�� | ���ˮ��Ӧ��������ˮ��Ӧ���� | |
| B�� | ��ԭ�ԣ�K��Na��Li����K���Դ�NaCl��ˮ��Һ���û��������� | |
| C�� | Li��Na��K��Rb��Cs�ȼ�������ʶ�������ɫ | |
| D�� | ���ԣ�LiOH��NaOH��KOH |
| A�� | NCl3�ĵ���ʽ��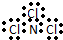 | B�� | H- �Ľṹʾ��ͼ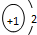 | ||
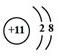
| C�� | ����4�����ӵ��ԭ�ӣ�74Li | D�� | ������Ľṹʽ��H-Cl-O |
| A�� | 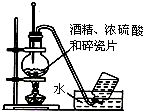 ʵ�������Ҵ���ȡ��ϩ | B�� | 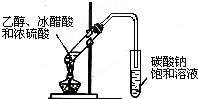 ʵ������ȡ�������� | ||
| C�� | 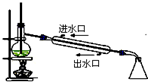 ʯ�ͷ��� | D�� | 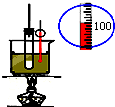 ʵ������ȡ������ |
| A�� | �ױ����������������ױ� | B�� | �ױ���ʹ���Ը��������Һ��ɫ | ||
| C�� | �ױ�ȼ�մ���Ũ��ĺ��� | D�� | �ױ��������������ӳɷ�Ӧ |

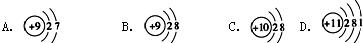
 ��
��