��Ŀ����
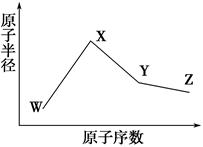
X��Y��Z���ֳ���Ԫ�صĵ��ʣ��ס��������ֳ����Ļ�����������ͼת����ϵ���ش��������⣺
��1�� ��X��̬ԭ����Χ�����Ų�ʽΪ3s2�������ɵڶ���������Ԫ�ص�ԭ�ӹ��ɵ���̬��������ӣ� Y��̬ԭ�ӵĹ����ʾʽΪ ���ĵ���ʽΪ
��2����Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2����������YΪ�ӷ���Һ�����ʡ���Ϊ��ɫ������ˮ�����塣��ZΪ �����Y��Ԫ�صĻ�̬ԭ�ӵĵ����Ų�ʽΪ ��
��3����X��Y��Ϊ�������ʣ�X��̬ԭ����Χ�����Ų�ʽΪ3s23p1����Ϊ���д��Եĺ�ɫ���壬��X���Ӧ�Ļ�ѧ����ʽΪ�� ��Y2+���ӵĵ����Ų�ʽΪ ��
��1��1s2 2s2
2s2 2p2
2p2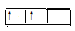 CO2�ĵ���ʽ
CO2�ĵ���ʽ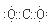
��2��H2��[Ar]3d104s24p5
��3��8Al+3Fe3O4 9Fe+ 4Al2O3 [Ar]3d6
9Fe+ 4Al2O3 [Ar]3d6
���������������1��X��̬ԭ����Χ�����Ų�ʽΪ3s2����X�ĺ��������Ϊ12������XΪþ����þ��Ӧ����̬������ӦΪ������̼�����Ϊ������̼��YΪ̼���ʡ�����Y�Ļ�̬ԭ�ӵĹ����ʾʽΪ1s2 2s2
2s2 2p2
2p2 ,������̼�ĵ���ʽΪ
,������̼�ĵ���ʽΪ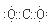
��2��Xԭ�ӵļ۵����Ų�Ϊns��n-1��np��n+2��,��n-1=2��n=3��X�����������Ų�Ϊ3s23p5��XΪ������������YΪ�ӷ���Һ�����ʣ���YΪҺ�壻��������Ϊ��ɫ������ˮ�����壬��Ϊ�Ȼ��⣬ZΪ����������35��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ[Ar]3d104s24p5
��3��X��̬ԭ����Χ�����Ų�ʽΪ3s23p1��XΪ�����ʣ���Ϊ���д��Եĺ�ɫ���壬��Ϊ������������X���Ӧ�����ȷ�Ӧ����ѧ����ʽΪ8Al+3Fe3O4 9Fe +4Al2O3 ��YΪ�����ʣ�Fe2+�ĵ����Ų�ʽΪ[Ar]3d6
9Fe +4Al2O3 ��YΪ�����ʣ�Fe2+�ĵ����Ų�ʽΪ[Ar]3d6
���㣺����Ԫ���ƶϼ���̬ԭ�ӵĺ�������Ų�ʽ�������ʾʽ��������ĵ���ʽ����ѧ����ʽ����д

 ��У����ϵ�д�
��У����ϵ�д��±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���Ԫ�ط��Ż�ѧʽ�ش��������⣺
| ���� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| �� | | | | | �� | | �� | |
| �� | �� | �� | �� | �� | | | �� | �� |
| �� | �� | | | | | | �� | |
��1������ЩԪ���У���������ǿ��Ԫ����
��2����ѧ��������õ�Ԫ����ԭ�ӽṹʾ��ͼΪ ��
��3��Ԫ�ص�����������Ӧ��ˮ������������ǿ���� ��������ǿ���� �������Ե����������� ��
��4���ڢۡ���Ԫ���У�ԭ�Ӱ뾶������ ��ԭ�Ӱ뾶��С���� ��
��5���ڢ����ĵ����У���ѧ���ʽϻ��õ��� ������ʲô��ѧ��Ӧ˵������ʵ��д����Ӧ�Ļ�ѧ����ʽ���� ��
���Ϊ�١����Ԫ�أ���Ԫ�����ڱ��е�λ�����£�
| ���� ���ڡ� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 1 | �� | | | | | | | �� |
| 2 | | | | �� | �� | �� | �� | |
| 3 | �� | �� | | | | �� | �� | |
�Իش��������⣺
(1)��ԭ��ֻҪ�γ�һ�Թ��õ��ӶԾʹﵽ���ȶ��ṹ��Ԫ����________��(��дԪ�ط���)
(2)�ٺܺ͢�Ԫ���γɵĻ�����Ļ�ѧʽΪ________���õ���ʽ��ʾ���γɹ���Ϊ________��
(3)���Ԫ�ص�����������ˮ����Ļ�ѧʽ��____________________��
(4)�١��ݡ��ߺ�Ԫ���γɵ�һ�ֻ�����ĵ���ʽ��________���ڸû������мȺ���________�����ֺ���________����

 ��
��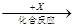 ���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ�������________(ѡ����ţ���ͬ)��
���������м��ǵ��ʣ��ҡ���Ϊ�����x�Ǿ��������Ե���ɫ���嵥�ʣ���Ļ�ѧ��ɲ�������________(ѡ����ţ���ͬ)��