��Ŀ����
��14�֣���һ�����ʵ���Ũ�ȵ�NaOH��Һ�ζ�10.00 mL��֪Ũ�ȵ����ᣬ�ζ������ͼ��ʾ���ش��������⣺
��1���йصζ��IJ����ɷֽ�Ϊ���¼���������������������NaOH��Һ��ϴ�ζ��ܡ���ȡNaOH��Һע���ʽ�ζ�������0���̶�����2~3 mL���� �ܵ���Һ������0����0���̶����£����¶���������ȡ10.00 mL����ע����ƿ�У������̪���ް���ƿ���ڵζ��ܵ����棬������������Һ�ζ������¶�����
��2�����ζ�ǰ���ӵζ��ܶ������ζ���ƽ�ӿ̶ȶ��������ʹ��������Ũ�ȵIJⶨ��� ���ƫ�ߡ���ƫ�͡����䡱����
��3�����÷�̪��ָʾ������ζ��յ��ʵ�������� ��
��4��c(HCl)= mol��L-1
��5��c(NaOH) = mol��L-1
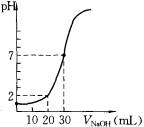
��6����ͼ��ʾ50 mL�ζ�����Һ���λ�ã����Һ�洦�Ķ�����a ����ζ�����Һ������������ţ� ��
��7�������£���0.01 mol?L-1H2SO4��Һ�ζ�0.01 mol?L-1 NaOH��Һ���кͺ��ˮ��100 ml�����ζ����յ�ʱ�ټ�һ��H2SO4����1��Ϊ0.05 ml�������ʱ��Һ��pHΪ ��

��1���йصζ��IJ����ɷֽ�Ϊ���¼���������������������NaOH��Һ��ϴ�ζ��ܡ���ȡNaOH��Һע���ʽ�ζ�������0���̶�����2~3 mL���� �ܵ���Һ������0����0���̶����£����¶���������ȡ10.00 mL����ע����ƿ�У������̪���ް���ƿ���ڵζ��ܵ����棬������������Һ�ζ������¶�����
��2�����ζ�ǰ���ӵζ��ܶ������ζ���ƽ�ӿ̶ȶ��������ʹ��������Ũ�ȵIJⶨ��� ���ƫ�ߡ���ƫ�͡����䡱����

��3�����÷�̪��ָʾ������ζ��յ��ʵ�������� ��
��4��c(HCl)= mol��L-1
��5��c(NaOH) = mol��L-1
��6����ͼ��ʾ50 mL�ζ�����Һ���λ�ã����Һ�洦�Ķ�����a ����ζ�����Һ������������ţ� ��
| A����a mL | B���ǣ�50��a��mL |
| C��һ������a mL | D��һ�����ڣ�50��a��mL |
��1�����ڵζ��ܼ���ʹ֮������Һ��2��ƫ��
��3�����һ�ε������ƿ����Һǡ�ó��ֺ�ɫ���Ұ�����ں�ɫ����ȥ
��4��0.09 ��5��0.03 ��6��D ��7��9
��3�����һ�ε������ƿ����Һǡ�ó��ֺ�ɫ���Ұ�����ں�ɫ����ȥ
��4��0.09 ��5��0.03 ��6��D ��7��9
��1����ʽ�ζ�����װ���Һ����Ҫ���ڵζ��ܼ���ʹ֮������Һ��
��2���ζ������϶��¿̶�����������ģ����Եζ�ǰ���ӵζ��ܶ�����������ƫС�ģ������������Ƶ����ƫ�����ƫ�͡�
��3���������������Ƶζ����ᣬ�����յ�ʱ�����������һ�ε������ƿ����Һǡ�ó��ֺ�ɫ���Ұ�����ں�ɫ����ȥ��
��4������ͼ���֪��������2ml��������ʱ����Һ��pH��1���յ�ʱ�����������Ƶ������30mol�����������Ũ�����������Ƶ�3������ �����x��0.03mol/L�����������Ũ����0.09mol/L��
�����x��0.03mol/L�����������Ũ����0.09mol/L��
��5������4��
��6�����ڵζ��ܵ����¶��Dz����̶��ߵģ����Եζ�������Һ�����Ӧ���ڣ�50��a��mL��
��7��һ�������������ӵ����ʵ�����0.05 ml��10��3��2��0.01 mol/L��10��6mol/L�����Թ���������������10��6mol/L�����c(OH��)��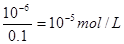 ,��������Ũ����10��9mol/L��pH����9.
,��������Ũ����10��9mol/L��pH����9.
��2���ζ������϶��¿̶�����������ģ����Եζ�ǰ���ӵζ��ܶ�����������ƫС�ģ������������Ƶ����ƫ�����ƫ�͡�
��3���������������Ƶζ����ᣬ�����յ�ʱ�����������һ�ε������ƿ����Һǡ�ó��ֺ�ɫ���Ұ�����ں�ɫ����ȥ��
��4������ͼ���֪��������2ml��������ʱ����Һ��pH��1���յ�ʱ�����������Ƶ������30mol�����������Ũ�����������Ƶ�3������
 �����x��0.03mol/L�����������Ũ����0.09mol/L��
�����x��0.03mol/L�����������Ũ����0.09mol/L����5������4��
��6�����ڵζ��ܵ����¶��Dz����̶��ߵģ����Եζ�������Һ�����Ӧ���ڣ�50��a��mL��
��7��һ�������������ӵ����ʵ�����0.05 ml��10��3��2��0.01 mol/L��10��6mol/L�����Թ���������������10��6mol/L�����c(OH��)��
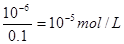 ,��������Ũ����10��9mol/L��pH����9.
,��������Ũ����10��9mol/L��pH����9.
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

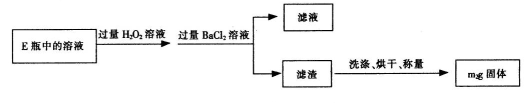
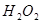 �ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����
�ֽⷴӦ���ʵ�Ӱ�죬ijͬѧ����֧�Թ��зֱ����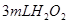 ��Һ���������ռ�
��Һ���������ռ�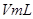 ���������ʱ�䣬ʵ���¼���£�
���������ʱ�䣬ʵ���¼���£�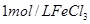
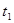
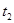
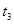
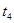
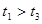 ��ԭ����__________��
��ԭ����__________��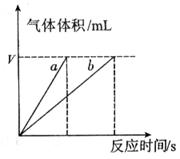
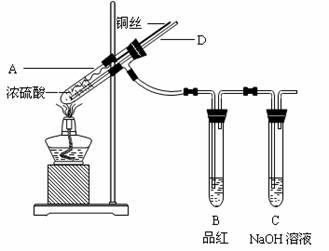
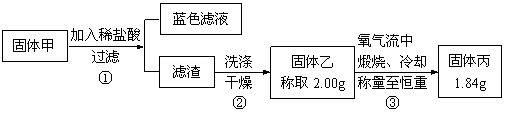 ��
��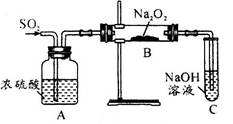
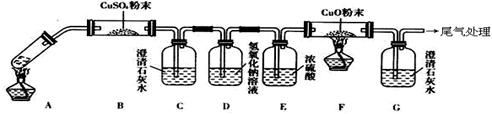
 CO2��+CO��+H2O
CO2��+CO��+H2O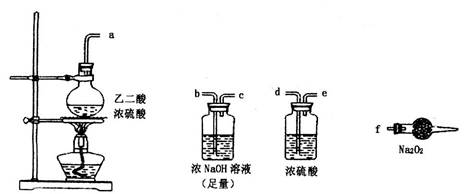
 l2����Ư���ԡ�
l2����Ư���ԡ�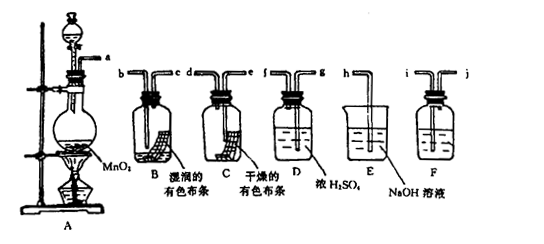
 ��
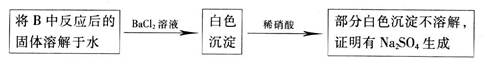
�� ��ɫ����������ɵó��Ľ�����
��ɫ����������ɵó��Ľ�����