��Ŀ����
16���Ȼ�ѧ����ʽ����1���ڱ�״���µ�11.2L������ȫȼ������CO2 ��Һ̬ˮ�ų�444.8KJ������298K�������Ȼ�ѧ����ʽΪCH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-889.6KJ/mol
��2����֪��������1mol��ѧ����Ҫ���յ������ֱ�Ϊ��C=O��745KJ/mol��O=O��496kJ/mol��C-H��414KJ/mol �����1molH-O����Ҫ�ṩ������Ϊ511.9kJ��
���� ��1������n=$\frac{V}{{V}_{m}}$�����������ʵ�������������1mol����ȼ�յķ�Ӧ�ȣ�����д����Ӧ���Ȼ�ѧ����ʽ��
��2�����ݷ�Ӧ�ȡ�H=��Ӧ���ܼ���-�������ܼ��ܣ���Ϸ�Ӧ�Ⱥͼ��ܼ��㣮
��� �⣺��1����״����11.2L��������ʵ���Ϊ$\frac{11.2L}{22.4L/mol}$=0.5mol��
��1mol������ȫȼ������Һ̬ˮ�ų�������Ϊ��2��444.8kJ=889.6kJ��
���Լ���ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-889.6KJ/mol��
�ʴ�Ϊ��CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-889.6KJ/mol��
��2�������1molH-O����Ҫ�ṩ������ΪQkJ����414��4+496��2-745��2-Q��4=-889.6�����Q=511.9��
�ʴ�Ϊ��511.9��
���� ���⿼���Ȼ�ѧ����ʽ����д����Ӧ���뻯ѧ��������������ϵ���ѶȲ���ע�����֪ʶ�����������գ�

��ϰ��ϵ�д�
�����Ŀ
6����ʹ0.01mol/L��NaHCO3��Һ�У�c��H+����c��CO32-����c��HCO3-�������٣��䷽���ǣ�������
| A�� | ͨ��CO2���� | B�� | ����NaCl���� | C�� | ����NaOH���� | D�� | ����Ba��OH��2���� |
7�����й��̻�����������ˮ���ص��ǣ�������
| A�� | �ȵĴ�����Һȥ����Ч���� | |
| B�� | ��TiCl4���ڴ���ˮ��ͬʱ���ȣ��ɵ�TiO2 | |
| C�� | ��Na2CO3��Һ�����ᴦ��ˮ���е�CaSO4 | |
| D�� | FeCl3��Һ���Ⱥ���ɫ���� |
11�����й��������˵����ȷ���ǣ�������
| A�� | ������ֻ�ܴ����ھ����� | |
| B�� | ��λ��ֻ���Ƕ�ԭ�ӷ��� | |
| C�� | �������ȶ�����ָ�����������������Ƿ��ֽ� | |
| D�� | Ѫ�����е�Fe2+��CO�γɵ���������O2�γɵ�������ȶ� |
5������ʽΪC7H16O�ı���һԪ��������ȥ��Ӧʱ�����Եõ����ֵ�ϩ������ô��Ľṹ��ʽ�ǣ�������
| A�� | 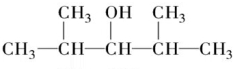 | B�� | 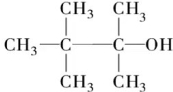 | ||
| C�� |  | D�� | CH3��CH2��5CH2OH |
1������˵������ȷ���ǣ�NA��ʾ�����ӵ���������������
| A�� | 60gSiO2�������2NA��Si-O�� | |
| B�� | 18D2O����ˮ����ȫ��⣬ת��2NA������ | |
| C�� | 1Llmol•L-1 Na2CO3��Һ��CO32-��ΪNA | |
| D�� | 16g���麬�Ц���ĿΪ4NA |
 ������Ԫ�����ڱ��е�λ���ǵ�4���ڢ�A�壬��������������Ӧˮ����Ļ�ѧʽ��Ca��OH��2��
������Ԫ�����ڱ��е�λ���ǵ�4���ڢ�A�壬��������������Ӧˮ����Ļ�ѧʽ��Ca��OH��2��