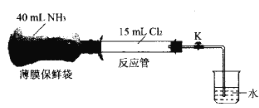
��Ŀ����
����Ŀ���±��Dz�ͬ�¶���ˮ�����ӻ�������
�¶�/�� | 25 | t1 | t2 |
ˮ�����ӻ����� | 1��10��14 | a | 1��10��12 |
�Իش��������⣺
(1)��25<t1<t2����Kw________1��10��14(����>������<����������)�������жϵ�������___________________��
(2)t2��ʱ����pH=11�Ŀ�������ҺV1 L��pH=1��ϡ����V2 L���(���Ϻ���Һ�����Ϊԭ����Һ���֮��)�����û����Һ��pH=2����V1��V2=________������Һ�и������ӵ�Ũ���ɴ�С��˳����________��
���𰸡�> �¶����ߣ�ˮ�ĵ���̶���������ˮ�����ӻ��������� 9��11 c(Na+)>c(SO42-)>c(H+)>c(OH-)
��������
(1)ˮ��������ʣ�����������������������¶ȶԻ�ѧƽ���ƶ���Ӱ�������
(2)���¶���ˮ�����ӻ�ΪKw=1��10-12����ͼ��ϣ������û��Һ��pH=2���������������c(H+)=![]() ���㣬��ʽ����pH���ɼ�������ȡ�
���㣬��ʽ����pH���ɼ�������ȡ�
(1)ˮ��һ�ּ����ĵ���ʣ����ڵ���ƽ��H2O![]() H++OH-�������¶ȣ��ٽ�ˮ�ĵ��룬ʹˮ�ĵ���ƽ�������ƶ���ˮ���������c(H+)��c(OH-)����������ˮ�����ӻ�Kw= c(H+)��c(OH-)Ҳ��������Kw>1��10��14��
H++OH-�������¶ȣ��ٽ�ˮ�ĵ��룬ʹˮ�ĵ���ƽ�������ƶ���ˮ���������c(H+)��c(OH-)����������ˮ�����ӻ�Kw= c(H+)��c(OH-)Ҳ��������Kw>1��10��14��
(2)���¶���ˮ�����ӻ�ΪKw=1��10-12����pH=11�Ŀ�����������������Ũ��Ϊ��c(OH-)=0.1mol/L��pH=1��ϡ������������Ũ��Ϊ0.1mol/L��pH=2����Һ��������Ũ��Ϊ0.01mol/L������Һ�����㣺0.1mol/L��V2-0.1mol/L��V1=0.01mol/L��(V1+V2)��
�����ɵã�V1��V2=9��11��
pH=11��NaOH��Һ�����ʵ���Ũ��Ϊ0.1mol/L�������Ϊ9L����ӦNaOH�����ʵ���Ϊ0.9mol����Һ��������Ϊ0.9mol��
pH=1��������Һ��Ũ��Ϊ0.05mol/L�������Ϊ11L����Ӧ����Һ������������ʵ���Ϊ0.55mol��
���ڷ�Ӧ����Һ��pH=2����Ӧ������Һ��c(H+)=0.01mol/L����Ӧ������Һ�����Ϊ20L����n(H+)=0.2mol��������Һ�����ԣ���Һ�е������������ʵ�����С����ͬһ��Һ�У���Һ�������ͬ��������Һ���������ʵ���Խ�࣬����c=![]() ��֪������Ũ�Ⱦ�Խ����˸���Һ�е�����Ũ�ȴ�С��ϵΪ��c(Na+)>c(SO42-)>c(H+)>c(OH-)��
��֪������Ũ�Ⱦ�Խ����˸���Һ�е�����Ũ�ȴ�С��ϵΪ��c(Na+)>c(SO42-)>c(H+)>c(OH-)��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����һ�����İ�������粒�������ij�ݻ��㶨����������У�������Ӧ��H2NCOONH4(s)![]() 2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��
2NH3(g)��CO2(g)���ڲ�ͬ�¶��£��÷�Ӧ��ƽ��״̬ʱ�IJ������������ʾ������˵����ȷ���ǣ� ��
�¶� | ƽ��Ũ��(mol��L��1) | |
c(NH3) | c(CO2) | |
T1 | 0.1 | |
T2 | 0.1 | |
A.��T2��T1����÷�Ӧ����H��0
B.���������N2��H2NCOONH4��������
C.NH3�����������ʱ��˵���÷�Ӧ�ﵽƽ��
D.T1��T2ʱ��ת����H2NCOONH4�����ʵ�����n(T2)��2��n(T1)
����Ŀ������ѡ���е�ԭ������������Ӧ����
ѡ�� | ���� | ԭ������ |
A | ��H2O2�м���MnO2���ܼ���H2O2�ķֽ����� | MnO2�����˷�Ӧ����Ļ�� |
B | ���ܱ��������з�Ӧ��A+xB��g�� | A�����塢x��1 |
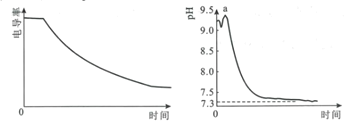
C | ��ʢ��NO2������ܱ�������������ˮ�У�������������ɫ���� | 2NO2��g�� |
D | ��5mL 0.005mol��L-1 FeCl3��Һ�м���5mL 0.015mol��L-1 KSCN��Һ����Һ�ʺ�ɫ���ٵμӼ���1 mol��L-1 KSCN��Һ����Һ��ɫ���� | ����Ӧ��Ũ�ȣ�ƽ��������Ӧ�����ƶ� |
A. A B. B C. C D. D