��Ŀ����
����������Ԫ��A��B��C��D��E��ԭ�����������������ǵ�ԭ�Ӻ�����Ӳ���֮��Ϊ10��D��E�����ʷ�Ӧ�����������ֲ�ͬ�����ӻ������A��B����Ԫ����ɵ�����QΪԭ�Ӹ�����Ϊ1��1��ֱ���η��ӡ�
��1��CԪ��Ϊ�������� ��Q�ĽṹʽΪ�������� ��
��2����A��B��D��E����Ԫ����ɵĻ���������϶࣬������Է���������С�����ʵĻ�ѧʽΪ�������� ��
��3��A��C��D��E����Ԫ��ԭ�ӵİ뾶�ɴ�С�Ĺ�ϵΪ������������������������ ��
��4�����������£���m mol C2��nmol A2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��C2 (g) + 3 A2(g)���� ![]() �� 2CA3(g)
�� 2CA3(g)
�ٷ�Ӧ���е�ijʱ��tʱ��C2��CA3 �����ʵ����ֱ�Ϊ15mol��6mol�� m��ֵΪ�������� ��
�ڷ�Ӧ��ƽ��ʱ�������������Ϊ896L(�����)������CA3�ĺ���(�������)Ϊ30%��ƽ��ʱCA3�����ʵ���Ϊ�������� ��
��ƽ���������У�n(C2)��n(A2)��n(CA3) = ���� ��
��1��N������C2H2�ĵ���ʽ��
��2��CH3ONa
��3��E>C>D>A
��4����18mol, ��12mol, ��3:4:3

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
�����Ŀ
����������Ԫ��A��B��C��D��ԭ��������������A��Cԭ���������8��A��B��C����Ԫ��ԭ�ӵ�����������֮��Ϊ15��Bԭ����������������Aԭ��������������һ�룮����������ȷ���ǣ�������
| A��ԭ�Ӱ뾶��A��D��C��B | B��B��C��D�ֱ���A�γɵĻ�����һ��������ͬ�Ļ�ѧ�� | C������������Ӧˮ��������ԣ�D��C | D�������£�����B�ܴ�������Ũ������ |
����������Ԫ��A��B��C��ԭ���������ε�����A��Cͬ���壬Bԭ�ӵ���������������Aԭ�ӵĴ���������������ԭ�ӵ�����������֮��Ϊ10��������������ȷ���ǣ�������
| A��ԭ�ڰ뾶A��B��C | B��A����̬�⻯���ȶ��Դ���C����̬�⻯���ȶ��� | C��A��C��Ԫ����������������ˮ���ϵõ���Ӧ���� | D������ʱ��A���ʿ��Դ�C�����������û��õ�C���� |
����������Ԫ��A��B��C��D��E��ԭ������������������A��Cͬ���壬A������Ԫ�ز���ͬһ���ڣ�B��Dͬ���壬������D�ĵ���Ϊ����ɫ���壮�����ƶ�����ȷ���ǣ�������
| A��ԭ�Ӱ뾶��С�����˳��r��C����r��D����r��E�� | B��Ԫ��D��E�ֱ���A�γɵĻ���������ȶ��ԣ�E��D | C��Ԫ��D������������Ӧˮ��������Ա�E��ǿ | D��Ԫ��B�ֱ���A��C�γɵĻ������л�ѧ����������ȫ��ͬ |
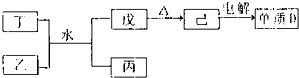
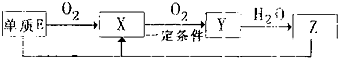
 ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2