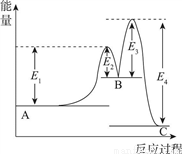
��Ŀ����
80 ��ʱ��2 L �ܱ������г���0.40 mol N2O4��������ӦN2O4 2NO2 ��H����Q kJ��mol-1��Q��0���������������:�����ж���ȷ����
2NO2 ��H����Q kJ��mol-1��Q��0���������������:�����ж���ȷ����
ʱ��/s | 0 | 20 | 40 | 60 | 80 | 100 |
c(NO2)/mol��L-1 | 0.00 | 0.12 | 0.20 | 0.26 | 0.30 | 0.30 |
A. �����¶ȸ÷�Ӧ��ƽ�ⳣ��K��С
B. 20��40 s �ڣ�v��N2O4����0.002 mol/L .s
C. ��Ӧ��ƽ��ʱ�����յ�����Ϊ0.30 Q kJ/mol
D. 100s ʱ��ͨ��0.40 mol N2O4������ƽ��ʱN2O4��ת���ʼ�С
��������ʵ��������������ó��Ľ��۴������
ѡ�� | �� �� | �� �� | �� �� |
A | �������м���Ũ���� | ���DZ�����ɶ�ĺ���״̿�����ų����ݼ�����ζ������ | Ũ���������ˮ�Ժ�ǿ������ |
B | ��ʢ��H2O2��Һ���Թ��м��뼸���ữ������������Һ | ��Һ����ػ�ɫ,һ��ʱ�����Һ�г������ݣ� ����к��ɫ�������� | Fe2+��H2O2�ֽ����O2��H2O2�ֽⷴӦ���ȣ��ٽ�Fe3+��ˮ��ƽ�������ƶ� |
C | ��Ƭ����ɰֽ��ĥ���ټ��� ��Ũ������ | ���������� | Ũ�������ǿ�����ԣ������£� ����Ũ����ۻ� |
D | ���Ũ�ȵ�KC1��KI���Һ���������AgNO3��Һ | �ȳ��ֻ�ɫ���� | Ksp(AgCl)>Ksp (AgI) |
A. A B. B C. C D. D