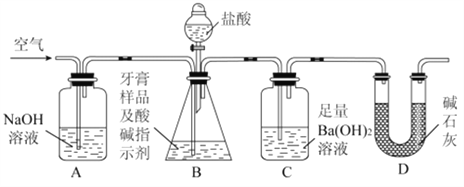
��Ŀ����
����Ŀ���й�ѧ����ˮú���任[CO(g)+H2O(g)=CO2(g)+H2(g) ��H]��ͻ���˵����¸�ת������߷�Ӧ���ʲ��ܼ�õ����⣬�ù����ǻ���˫���ܴ�������������ͬ���ӣ���ʵ�ֵġ���Ӧ����ʾ��ͼ���£�
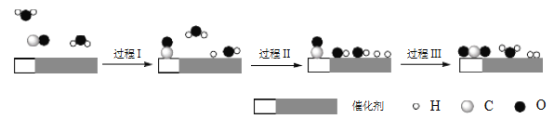
����˵����ȷ����
A. ���̢��̢��Ϊ���ȹ���
B. ���̢������˾��м��Թ��ۼ���H2��CO2
C. ʹ�ô���������ˮú���任��Ӧ�Ħ�H
D. ͼʾ�����е�H2O�������˷�Ӧ����
���𰸡�D
��������A. ���ݷ�Ӧ����ʾ��ͼ�����̢���ˮ�����еĻ�ѧ�����ѵĹ��̣�Ϊ���ȹ��̣���A����B. ���̢���CO������ԭ���ź���ԭ���γ��˶�����̼��ˮ����������H2�еĻ�ѧ��Ϊ�Ǽ��Լ�����B����C.�������ܸı䷴Ӧ����H����C����D. ���ݷ�Ӧ����ʾ��ͼ�����̢���ˮ�����еĻ�ѧ�����ѣ����̢�Ҳ��ˮ�����еĻ�ѧ�����ѵĹ��̣����̢����γ���ˮ���ӣ����H2O�������˷�Ӧ���̣���D��ȷ����ѡD��

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ