��Ŀ����
I.S4N4�Ľṹ��ͼ:

��1��S4N4�ľ���������___��
��2���ø���İ�������S2Cl2��CCl4,��Һ�п���S4N4����ѧ��Ӧ����Ϊ:6S2Cl2��16NH3��S4N4��S8��12NH4Cl
��������Ӧ�����У�û���ƻ����γɵ�������������___��
a���Ӽ���b���Լ���c�Ǽ��Լ���d��������e��λ����f���»���
��S2Cl2�У�Sԭ�ӹ�����ӻ�������___��
II.���ʰ����ͭ(II)�����类���ֵĵ����������Σ����Ľṹ��ͼ��

��3����̬Cu2+����Χ�����Ų�ʽΪ_ _��
��4�����ʰ����ͭ( II)�У���һ����������Ԫ����縺����С�ķǽ���Ԫ�ؿ��γɶ�����������һ����5��10���ӵ����������Ŀռ乹���� _��
��5��lmol���ʰ����ͭ(II)���еĶ�����Ŀ�� _ ��
��6�����ʰ����ͭ(II)�ṹ�У���ͭ�γɵĻ�ѧ����һ��������λ������ ____ (��д���)��

��1��S4N4�ľ���������___��
��2���ø���İ�������S2Cl2��CCl4,��Һ�п���S4N4����ѧ��Ӧ����Ϊ:6S2Cl2��16NH3��S4N4��S8��12NH4Cl
��������Ӧ�����У�û���ƻ����γɵ�������������___��
a���Ӽ���b���Լ���c�Ǽ��Լ���d��������e��λ����f���»���
��S2Cl2�У�Sԭ�ӹ�����ӻ�������___��
II.���ʰ����ͭ(II)�����类���ֵĵ����������Σ����Ľṹ��ͼ��

��3����̬Cu2+����Χ�����Ų�ʽΪ_ _��
��4�����ʰ����ͭ( II)�У���һ����������Ԫ����縺����С�ķǽ���Ԫ�ؿ��γɶ�����������һ����5��10���ӵ����������Ŀռ乹���� _��
��5��lmol���ʰ����ͭ(II)���еĶ�����Ŀ�� _ ��
��6�����ʰ����ͭ(II)�ṹ�У���ͭ�γɵĻ�ѧ����һ��������λ������ ____ (��д���)��
��1�����Ӿ��壨1�֣�
��2���� d ��2�֣���sp3 ��2�֣�
��3��3d9 ��2�֣�
��4�����������Σ�2�֣�
��5��2NA��1.204��1024 ��2�֣�
��6��1��4 ��2�֣�
��2���� d ��2�֣���sp3 ��2�֣�
��3��3d9 ��2�֣�
��4�����������Σ�2�֣�
��5��2NA��1.204��1024 ��2�֣�
��6��1��4 ��2�֣�
�����������1��)S��N���ǻ��õķǽ�������S4N4�Ľṹʽ��֪���ṹ�д��ڷ��ӣ�����γɵľ��������Ƿ��Ӿ��塣
��2�����ڷ�Ӧ6S2Cl2��16NH3��S4N4��S8��12NH4Cl�����У���Ӧ���еĻ�ѧ���м��Լ��ͷǼ��Լ����Ҷ����γɵľ��嶼�Ƿ��Ӿ��壬�Ұ����д������������Ӧ�����й��ۼ������Ӽ��γɣ��Ȼ�������ӻ����������λ��������û���ƻ����γɵ������������ǽ���������ѡd��
��S2Cl2�ṹʽΪCl��S��S��Cl������ÿ��Sԭ���γ�2�����ۼ���ͬʱ������2�Թ¶Ե��ӣ���Sԭ�Ӽ۲���Ӷ�����4������Sԭ�ӹ�����ӻ�������sp3�ӻ���
��3��ͭԪ�ص�ԭ��������29������ݺ�������Ų����ɿ�֪ͭԪ�صĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1�����̬Cu2+����Χ�����Ų�ʽΪ3d9��
��4�����ڵ�Ԫ�ص�2p�㶫���Ӵ��ڰ����״̬���ȶ���ǿ����˵�Ԫ�صĵ�һ�����ܴ�����Ԫ�صĵ�һ�����ܣ����Զ��ʰ����ͭ( II)�У���һ����������Ԫ���ǵ�Ԫ�أ��縺����С�ķǽ���Ԫ������Ԫ�ء������γɶ�����������һ����5��10���ӵ�����������NH4�������е�Ԫ�ؼ۲���Ӷ�����4���Ҳ����ڹ¶Ե��ӣ����Կռ乹�������������Ρ�
��5�����ݽṹʽ��֪�������д���2��̼��˫��������lmol���ʰ����ͭ(II)���еĶ�����Ŀ��2NA��1.204��1024��
��6������������ͭ���ܽ��ڰ�ˮ���γ���λ������˵��ͭ�������뵪Ԫ���γ���λ�������Զ��ʰ����ͭ(II)�ṹ�У���ͭ�γɵĻ�ѧ����һ��������λ������1��4��

��ϰ��ϵ�д�
�����Ŀ
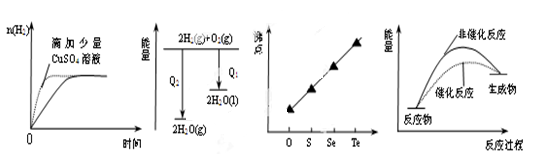

 �������ʵ���Ϊ mol��
�������ʵ���Ϊ mol��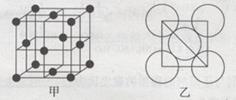
 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____HIO4�����������������������
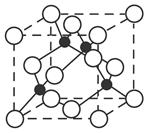

 (PAN)�ȶ�����Ⱦ�
(PAN)�ȶ�����Ⱦ�