��Ŀ����
��������(ClO2)����������(NaClO2)��Ϊ��ЧƯ����������ij��ѧ��ȤС��ͬѧ�����Ʊ�չ���о�������������֪���ٶ�������(ClO2)��Cl2�����ʾ���һ�������ԣ�
��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣

��ش��������⣺
(1)��������a������___________��װ��B��������___________________��
(2)װ��C���Ʊ�NaClO2�Ļ�ѧ����ʽΪ________________________��ͨ��������K��Ũ����ĵ��ٿ����������٣���Ӧʹ����C�������ٶȽ�________(��족������)��װ��D��������_______________________________��
(3)��װ��C��Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ�
��________________���ܵ���60�����õ���Ʒ��
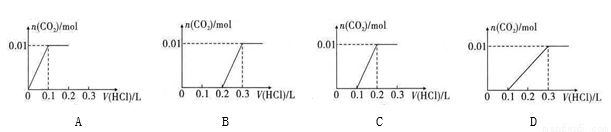
(4)Ϊ�ⶨij����������Ʒ�Ĵ����������ʵ�鷽����������ʵ�飺
��һ����ȷ��ȡ��������������Ʒmg��С�ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO +4I
+4I +4H+==2H2O+2I2+Cl
+4H+==2H2O+2I2+Cl )�������û��Һ���250mL������Һ��
)�������û��Һ���250mL������Һ��
�ڶ�������ȡ25.00mL������Һ����ƿ�У��Ӽ��ε�����Һ����cmol��L-1Na2S2O3����Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪVmL(��֪��I2+2S2O32��==2I +S4O52��)��
+S4O52��)��
�ٴﵽ�ζ��յ�ʱ������Ϊ_______________________________��
�ڸ���Ʒ��NaClO2����������Ϊ_______________(�ú�m��c��V�Ĵ���ʽ��ʾ)��
���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ���ԭ�������ӷ���ʽ��ʾΪ_______________________��