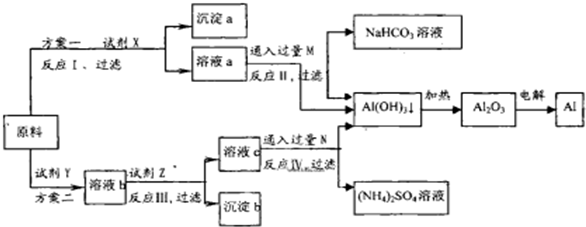
��Ŀ����
ʵ��������480mL 0.1mol/L������������Һ����ش��������⣺
��1������
��2������ʱӦѡ��
��3���ڴ����ƹ����н�ͷ�ιܵ�������
��4����ʵ��ʱ�����������������ʹ�������ҺŨ�ȷ���ʲô�仯���ƫ�ߡ���ƫ�͡�����Ӱ�족����
A����ˮ����ʱ�����̶���
B��û�н�ϴ��Һת������ƿ��
C������ƿ�ڱ�մ��ˮ��û�к�ɾͽ�������
D������ʱ����
��5��������5%������������Һ��ȣ�
���õ�����ͬ������
���õ��IJ�ͬ������
��1������
С�ձ����������
С�ձ����������
g�������ƹ���ʱӦ�����ձ�
�ձ�
�н��У���2������ʱӦѡ��
500mL
500mL
����ƿ����3���ڴ����ƹ����н�ͷ�ιܵ�������
�μ�ˮ����Һ����͵���̶�������
�μ�ˮ����Һ����͵���̶�������
����4����ʵ��ʱ�����������������ʹ�������ҺŨ�ȷ���ʲô�仯���ƫ�ߡ���ƫ�͡�����Ӱ�족����
A����ˮ����ʱ�����̶���
ƫ��
ƫ��
��B��û�н�ϴ��Һת������ƿ��
ƫ�ͣ�
ƫ�ͣ�
��C������ƿ�ڱ�մ��ˮ��û�к�ɾͽ�������
ƫ��
ƫ��
��D������ʱ����
ƫ��
ƫ��
����5��������5%������������Һ��ȣ�
���õ�����ͬ������
������ƽ �ձ� ������ ��ͷ�ι�
������ƽ �ձ� ������ ��ͷ�ι�
�����õ��IJ�ͬ������
��Ͳ��500mL����ƿ
��Ͳ��500mL����ƿ
����������1���������������׳��⣬����ڲ��������г����������ձ����ܽ⣻
��2��������Һ�����480mL��������ƿ�Ĺ��û��480mL��
��3�����ݶ���ʱ��Һ���̶���1-2cmʱ���ý�ͷ�ιܵμ�ˮ��
��4������c=
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��5����������һ�����ʵ���Ũ����Һ�����г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ó�������������������5%������������Һ�IJ��裺���㡢��������ȡ���ܽ⣬�ó�����������
��2��������Һ�����480mL��������ƿ�Ĺ��û��480mL��
��3�����ݶ���ʱ��Һ���̶���1-2cmʱ���ý�ͷ�ιܵμ�ˮ��
��4������c=
| n |
| V |
��5����������һ�����ʵ���Ũ����Һ�����г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ó�������������������5%������������Һ�IJ��裺���㡢��������ȡ���ܽ⣬�ó�����������
����⣺��1�������������׳��⣬����ڲ��������г����������ձ����ܽ⣬�ʴ�Ϊ��С�ձ�����������ձ���
��2������Һ�����480mL��������ƿ�Ĺ��û��480mL������ѡ��500mL����ƿ���ʴ�Ϊ��500mL��
��3����Һ���̶���1-2cmʱ���ý�ͷ�ιܵμ�ˮ���ʴ�Ϊ���μ�ˮ����Һ����͵���̶������У�
��4��A����ˮ����ʱ�����̶��ߣ���Һ�����ƫ��Ũ��ƫС���ʴ�Ϊ��ƫ�ͣ�
B��û�н�ϴ��Һת������ƿ�У����ʵ��������٣�Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
C������ƿ�ڱ�մ��ˮ��û�к�ɾͽ������ƣ���Ӱ�죬Ũ�Ȳ��䣬�ʴ�Ϊ����Ӱ�죻
D������ʱ���ӣ���Һ�����ƫС��Ũ��ƫ�ʴ�Ϊ��ƫ�ߣ�
��5����������һ�����ʵ���Ũ����Һ�����г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
����5%������������Һ�IJ��裺���㡢��������ȡ���ܽ⣬�ȼ������������Ϊ��90��10%=9�ˣ�ˮ������Ϊ90-9=81�ˣ���Կ��ȡҩƷ������ƽ����ҩƷ��Ҫ�õ�ҩ�ף������ձ��ڣ�����Ͳ����Ҫ�õ���ͷ�ιܣ�ȡˮ�����ձ��У��ò��������裮������Ҫ������Ϊ����ƽ������ҩ�ס���Ͳ����ͷ�ιܡ��ձ�����������
���õ�����ͬ����Ϊ������ƽ��ҩ�ס��ձ�������������ͷ�ιܣ��ʴ�Ϊ��������ƽ��ҩ�ס��ձ�������������ͷ�ιܣ�
���õ��IJ�ͬ����Ϊ��Ͳ��500mL����ƿ���ʴ�Ϊ����Ͳ��500mL����ƿ��
��2������Һ�����480mL��������ƿ�Ĺ��û��480mL������ѡ��500mL����ƿ���ʴ�Ϊ��500mL��
��3����Һ���̶���1-2cmʱ���ý�ͷ�ιܵμ�ˮ���ʴ�Ϊ���μ�ˮ����Һ����͵���̶������У�
��4��A����ˮ����ʱ�����̶��ߣ���Һ�����ƫ��Ũ��ƫС���ʴ�Ϊ��ƫ�ͣ�
B��û�н�ϴ��Һת������ƿ�У����ʵ��������٣�Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
C������ƿ�ڱ�մ��ˮ��û�к�ɾͽ������ƣ���Ӱ�죬Ũ�Ȳ��䣬�ʴ�Ϊ����Ӱ�죻
D������ʱ���ӣ���Һ�����ƫС��Ũ��ƫ�ʴ�Ϊ��ƫ�ߣ�
��5����������һ�����ʵ���Ũ����Һ�����г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
����5%������������Һ�IJ��裺���㡢��������ȡ���ܽ⣬�ȼ������������Ϊ��90��10%=9�ˣ�ˮ������Ϊ90-9=81�ˣ���Կ��ȡҩƷ������ƽ����ҩƷ��Ҫ�õ�ҩ�ף������ձ��ڣ�����Ͳ����Ҫ�õ���ͷ�ιܣ�ȡˮ�����ձ��У��ò��������裮������Ҫ������Ϊ����ƽ������ҩ�ס���Ͳ����ͷ�ιܡ��ձ�����������
���õ�����ͬ����Ϊ������ƽ��ҩ�ס��ձ�������������ͷ�ιܣ��ʴ�Ϊ��������ƽ��ҩ�ס��ձ�������������ͷ�ιܣ�
���õ��IJ�ͬ����Ϊ��Ͳ��500mL����ƿ���ʴ�Ϊ����Ͳ��500mL����ƿ��
������������Ҫ�������һ������������������Һ������������ƽ��ҩ�ס��ձ�����Ͳ����ͷ�ιܡ���������

��ϰ��ϵ�д�
�����Ŀ