��Ŀ����
��1�����ڼ�ͼʵ������Ԥ����ȷ����
A��ʵ������ã���Һ���ٷֲ㣬�ұ�����ɫ��
B��ʵ�����Ƭ������ȫ�ܽ⣬�Ҹ��������Һ����ɫ
C��ʵ�����ϡHNO3Ƭ�̣���Һ�������ݲ��������ƿ��ʼ�ձ�����ɫ
D��ʵ���������Һ�����ɫ��ֹͣ���ȣ��ù���ͨ����ϵʱ�ɲ������������
��2������ͭ��Ϳ�Ϲ�ҵ��ӡȾ��ҵ��ũҵ�ȷ�������ҪӦ��
��ʵ��������480ml 0.1mol/L��CuSO4��Һ������������ƽ��ȡ����
��ijͬѧ��Ҫ��ʵ�������Ʊ�����ͭ����ѯ���ϵ�֪��ͭм����ϡ�����в��ܽ⣬����ϡ�����м���H2O2��ͭм�����ܽ⣮Ϊ��֤��ʵ�飬ʵ��С�����ʵ��װ����ͼ�ң��÷�Ӧ�Ļ�ѧ����ʽ��

D
D
A��ʵ������ã���Һ���ٷֲ㣬�ұ�����ɫ��
B��ʵ�����Ƭ������ȫ�ܽ⣬�Ҹ��������Һ����ɫ
C��ʵ�����ϡHNO3Ƭ�̣���Һ�������ݲ��������ƿ��ʼ�ձ�����ɫ
D��ʵ���������Һ�����ɫ��ֹͣ���ȣ��ù���ͨ����ϵʱ�ɲ������������
��2������ͭ��Ϳ�Ϲ�ҵ��ӡȾ��ҵ��ũҵ�ȷ�������ҪӦ��
��ʵ��������480ml 0.1mol/L��CuSO4��Һ������������ƽ��ȡ����
12.5g
12.5g
g����ijͬѧ��Ҫ��ʵ�������Ʊ�����ͭ����ѯ���ϵ�֪��ͭм����ϡ�����в��ܽ⣬����ϡ�����м���H2O2��ͭм�����ܽ⣮Ϊ��֤��ʵ�飬ʵ��С�����ʵ��װ����ͼ�ң��÷�Ӧ�Ļ�ѧ����ʽ��
Cu+H2O2+H2SO4=CuSO4+2H2O
Cu+H2O2+H2SO4=CuSO4+2H2O
������H2O2��ϡ���������ƿ�е�˳��ߵ���ʵ��õ��Ľ�����ͭм����H2O2�в�������Ӧ������H2O2�м���ϡ���ᣬͭм�����ܽ�
ͭм����H2O2�в�������Ӧ������H2O2�м���ϡ���ᣬͭм�����ܽ�
��
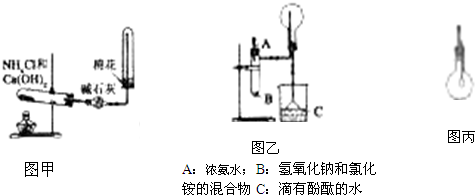
��������1��A��װ��ͼ�б�������ˮ��ˮ�ֲ㣻
B������������Ũ�����з����ۻ�����
C��ͭ��ϡ���ᷢ������һ�����������ڹ��ƿ�����������е�������Ӧ���ɺ���ɫ�������������
D�������Ȼ�����Һ���ȵ����ɫ���Ƶ������������壻
��2���ٸ���n=cv��������CuSO4�����ʵ���������CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������m=nM����CuSO4?5H2O��������
��˫��ˮ�������Ա�ϡ����ǿ�Ķ࣬���Խ�ͭ�������������ͭ��ϡ���ᷴӦ��
B������������Ũ�����з����ۻ�����
C��ͭ��ϡ���ᷢ������һ�����������ڹ��ƿ�����������е�������Ӧ���ɺ���ɫ�������������
D�������Ȼ�����Һ���ȵ����ɫ���Ƶ������������壻
��2���ٸ���n=cv��������CuSO4�����ʵ���������CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������m=nM����CuSO4?5H2O��������
��˫��ˮ�������Ա�ϡ����ǿ�Ķ࣬���Խ�ͭ�������������ͭ��ϡ���ᷴӦ��
����⣺��1��A��װ��ͼ�б�������ˮ��ˮ�ֲ㣬�����غ�ɫ����A����
B������������Ũ�����з����ۻ�������ֹ��Ӧ���У���Ƭ�����ܽ⣬���������Һ����ɫ����B����
C��ͭ��ϡ���ᷢ������һ�����������ڹ��ƿ�����������е�������Ӧ���ɺ���ɫ���������������C����
D�������Ȼ�����Һ���ȵ����ɫ���Ƶ������������壻������ж����������ͨ������һ��������ͨ·����D��ȷ��
�ʴ�Ϊ��D��
��2����������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml����ƿ��CuSO4�����ʵ���n=cV=0.5L��0.1mol?L-1=0.05mol��CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������CuSO4?5H2O������0.05mol��250g/mol=12.5g���ʴ�Ϊ��12.5��
����Ϊ˫��ˮ��������Һ���Ȱ�ͭ����������ͭ����Ȼ����һ�����ķ�Ӧ���γ�һ��ƽ�⣬�����γɵ�����ͭ���Ͼͻᱻϡ�����ܽ⣬ƽ�ⱻ���ƣ���Ӧ����������У��ʶ����ܽ⣬��Ӧ�Ļ�ѧ����ʽΪ��Cu+H2O2+H2SO4=CuSO4+2H2O���ʴ�Ϊ��Cu+H2O2+H2SO4=CuSO4+2H2O��
����H2O2��ϡ���������ƿ�е�˳��ߵ���ʵ��õ��Ľ����ǣ�ͭм����H2O2�в�������Ӧ������H2O2�м���ϡ���ᣬͭм�����ܽ⣻
�ʴ�Ϊ��ͭм����H2O2�в�������Ӧ������H2O2�м���ϡ���ᣬͭм�����ܽ⣻
B������������Ũ�����з����ۻ�������ֹ��Ӧ���У���Ƭ�����ܽ⣬���������Һ����ɫ����B����
C��ͭ��ϡ���ᷢ������һ�����������ڹ��ƿ�����������е�������Ӧ���ɺ���ɫ���������������C����
D�������Ȼ�����Һ���ȵ����ɫ���Ƶ������������壻������ж����������ͨ������һ��������ͨ·����D��ȷ��
�ʴ�Ϊ��D��
��2����������Һ�����Ϊ480ml��������ƿ�Ĺ��û��480ml��ֻ��ѡ��500ml����ƿ��CuSO4�����ʵ���n=cV=0.5L��0.1mol?L-1=0.05mol��CuSO4?5H2O�����ʵ�������CuSO4�����ʵ���������CuSO4?5H2O������0.05mol��250g/mol=12.5g���ʴ�Ϊ��12.5��
����Ϊ˫��ˮ��������Һ���Ȱ�ͭ����������ͭ����Ȼ����һ�����ķ�Ӧ���γ�һ��ƽ�⣬�����γɵ�����ͭ���Ͼͻᱻϡ�����ܽ⣬ƽ�ⱻ���ƣ���Ӧ����������У��ʶ����ܽ⣬��Ӧ�Ļ�ѧ����ʽΪ��Cu+H2O2+H2SO4=CuSO4+2H2O���ʴ�Ϊ��Cu+H2O2+H2SO4=CuSO4+2H2O��
����H2O2��ϡ���������ƿ�е�˳��ߵ���ʵ��õ��Ľ����ǣ�ͭм����H2O2�в�������Ӧ������H2O2�м���ϡ���ᣬͭм�����ܽ⣻
�ʴ�Ϊ��ͭм����H2O2�в�������Ӧ������H2O2�м���ϡ���ᣬͭм�����ܽ⣻
���������⿼���˳������ʵ���ȡԭ����װ�÷�����ʵ�鷽��������жϣ�ע��ʵ��Ļ�������������ע�����

��ϰ��ϵ�д�
�����Ŀ