��Ŀ����
12��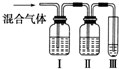 Ϊ̽����ϩ����ļӳɷ�Ӧ����ͬѧ��Ʋ�����������ʵ�飺�����Ҵ���Ũ����Ϊԭ����ȡ��ϩ�������ɵ�����ֱ��ͨ����ˮ�У�������Һ��ɫ����֤����ϩ����ˮ�����˼ӳɷ�Ӧ��
Ϊ̽����ϩ����ļӳɷ�Ӧ����ͬѧ��Ʋ�����������ʵ�飺�����Ҵ���Ũ����Ϊԭ����ȡ��ϩ�������ɵ�����ֱ��ͨ����ˮ�У�������Һ��ɫ����֤����ϩ����ˮ�����˼ӳɷ�Ӧ����ͬѧ�����ڼ�ͬѧ��ʵ���У������������д̼�����ζ���Ʋ����Ƶõ���ϩ�л����ܺ��������л�ԭ�Ե��������壬�ɴ�����������Ȱ����������ȥ��������ˮ��Ӧ��
����ش��������⣺
��1������ȡ��ϩ�Ļ�ѧ����ʽΪ��CH3CH2OH$��_{170��}^{Ũ����}$CH2�TCH2��+H2O��
����ϩ����ˮ�����ӳɷ�Ӧ�Ļ�ѧ����ʽΪ��CH2�TCH2+Br2-��CH2Br-CH2Br��
��2����ͬѧ��Ƶ�ʵ�鲻�ܣ���ܡ����ܡ�����֤��ϩ����ˮ�����˼ӳɷ�Ӧ����������AC��
A��ʹ��ˮ��ɫ�ķ�Ӧ��δ���Ǽӳɷ�Ӧ B��ʹ��ˮ��ɫ�ķ�Ӧ�����Ǽӳɷ�Ӧ
C��ʹ��ˮ��ɫ�����ʣ�δ������ϩ D��ʹ��ˮ��ɫ�����ʣ�������ϩ
��3����ͬѧ�Ʋ����ϩ�бض����е�һ������������SO2��������ˮ������Ӧ�����ӷ���ʽ��SO2+Br2+2H2O�T4H++SO22-+2Br-��
����֤�����б����ȥ�������壬Ϊ�ˣ���ͬѧ�������ͼ��ʾ��ʵ��װ�ã���ش𣺢�װ���п�ʢ�ŵ��Լ��ǣ���B����A����C��������ţ�
A��Ʒ����Һ B��NaOH��Һ C����ˮ D�����Ը��������Һ
��4��Ϊ��֤��һ��Ӧ�Ǽӳɷ�Ӧ������ȡ����Ӧ����ͬѧ�������pH��ֽ���ⶨ��Ӧ��Һ�����ԣ������ǣ��������ȡ����Ӧ���ض�����HBr����Һ���Խ���������ǿ���ʿ���pH��ֽ��֤��
���� ��1������Ũ������������170�������£��Ҵ�������ȥ��Ӧ������ϩ��ˮ��
����ϩ�к�̼̼˫����������ˮ�����ӳɷ�Ӧ����1��2-�������飻
��2���Ҵ���Ũ������ԭ����ȡ����ϩ�к��ж�����������ʣ�����������ʹ��ˮ��ɫ����ˮ��ɫ����֤������ϩ����ˮ�����˼ӳɷ�Ӧ��
��3���д̼�����ζ�������Ƕ��������������ܱ��������������Ҫ��֤��ϩ֮ǰ�ȳ�ȥ�����������Ƿ�������������������ˮ������ϩ��
��4���������ȡ����Ӧ�������廯�����ɣ���Һ��ǿ���ԣ�������ҺpHȷ��������Ӧ���ͣ�
��� �⣺��1������Ũ������������170�������£��Ҵ�������ȥ��Ӧ������ϩ��ˮ����Ӧ����ʽΪCH3CH2OH$��_{170��}^{Ũ����}$CH2�TCH2��+H2O��
�ʴ�Ϊ��CH3CH2OH$��_{170��}^{Ũ����}$CH2�TCH2��+H2O��
����ϩ�к�̼̼˫����������ˮ�����ӳɷ�Ӧ����1��2-�������飬����ʽΪ��CH2�TCH2+Br2-��CH2Br-CH2Br���ʴ�Ϊ��CH2�TCH2+Br2-��CH2Br-CH2Br��
��2���ô˷��õ�����ϩ�ڿ��ܺ���SO2���壬��SO2�ܽ���ˮ��ԭ��ʹ֮��ɫ������ʽΪSO2+Br2+2H2O�T2HBr+H2SO4����ˮ��ɫ����֤������ϩ����ˮ�����˼ӳɷ�Ӧ�ˣ�
�ʴ�Ϊ�����ܣ�AC��
��3����ϩ�п��ܺ��е�һ������������SO2��������ˮ������Ӧ�Ļ�ѧ����ʽ�ǣ�SO2+Br2+2H2O�T2HBr+H2SO4�����ӷ���ʽΪ��SO2+Br2+2H2O�T4H++SO22-+2Br-����֤�����б����ų���������ĸ��ţ������ȳ�ȥ��������I��ʢ������������Һ��ȥ��������II��ʢ��Ʒ����Һ������������Ƿ������III��ʢ����ˮ������ϩ�����ʣ�
�ʴ�Ϊ��SO2��SO2+Br2+2H2O�T4H++SO22-+2Br-��B��A��C��
��4������ϩ���巢��ȡ����Ӧ����÷�Ӧ�����廯�����ɣ���pH��ֽ�ⷴӦ����ҺӦ�ó����ԣ������Һû�г����ԣ���֤�������ӳɷ�Ӧ��
�ʴ�Ϊ���������ȡ����Ӧ���ض�����HBr����Һ���Խ���������ǿ���ʿ���pH��ֽ��֤��
���� ������Ҫ������ϩ���Ʊ�������ʵ�飬�ѶȲ���ע����ϩ�ij��Ӻ�����ʵ�飬������ϩ����ˮ��Ӧԭ����ͬ�IJ������ǽ���Ĺؼ���

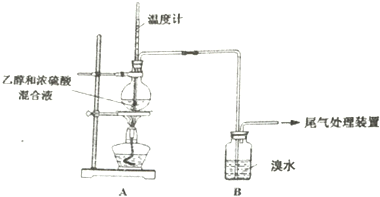
| ���� | ���� |
| ��ȼ�ƾ��ƣ� ������170�� | a��A����ƿ��Һ�彥����� b��B����������ð������Һ����ɫ |
| �� | |
| ʵ����� | c��A����ƿ�ڸ���������ɫ����״��д̼�����ζ�ݳ� |
��2������ʹB����Һ��ɫ�����ʣ�����Ϊ��C2H4������Ϊ�����ų�SO2�����ã�
�ٸ��ݼĹ۵㣬C2H4ʹB����Һ��ɫ�Ļ�ѧ����ʽΪCH2=CH2+Br2��CH2Br-CH2Br��
���Ҹ�������c��Ϊ������SO2��SO2ʹB����Һ��ɫ�Ļ�ѧ����ʽ��SO2+Br2+2H2O=2HBr+H2SO4��
��3��Ϊ֤ʵ���Թ۵㣬�ס���ͬѧ����ʵ�飬������A��B������һ��װ��ij���Լ���ϴ��ƿ�����۲쵽��ˮ��ɫ��
�ٸ��ݼ���ƣ�ϴ��ƿ��ʢ�ŵ��Լ���NaOH��Һ��
����Ϊ��һ����֤��۵㣬ȡ������Ӧ���B����Һ����������BaCl2��Һ��������������ɫ��������Ӧ�����ӷ���ʽΪSO2+2H2O+Br2�T4H++2Br-+SO42-��SO42-+Ba2+�TBaSO4����SO2+2H2O+Br2+Ba2+�T4H++2Br-+BaSO4����
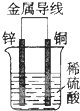
| A�� | ͭƬΪ���� | B�� | ͭƬ�������� | ||
| C�� | ���Ӵ�пƬ����������ͭƬ | D�� | ��������ͭƬ���汻���� |
| A�� | Mg2+?SO42-?K+?Cl- | B�� | K+?Cl-?NO-3?Cu2+ | ||
| C�� | Na+?SO2-4?H+?NO-3 | D�� | K+?CO32-?Cl-?Na+ |

����˵����ȷ���ǣ�������
| A�� | 1 mol N2��g���� 1 mol O2��g����Ӧ�ų�������Ϊ 180 kJ | |
| B�� | 1 mol N2��g���� 1 mol O2��g������������� 2 mol NO��g����������� | |
| C�� | ͨ������£�N2��g���� O2��g�������ֱ������ NO��g�� | |
| D�� | NO ��һ��������������� NaOH ��Һ��Ӧ�����κ�ˮ |
| A�� | ��Һ���������������ƶ� | |
| B�� | ��Һ�����������ʵ������� | |
| C�� | �����ĵ缫��Ӧʽ�ǣ�N2H4+4OH--4e-�T4H2O+N2�� | |
| D�� | �����ĵ缫��Ӧʽ�ǣ�O2+4H++4e-�T2H2O |
Ӧ�ɱ�ʾΪ��5MnO2+2Ag+2NaCl�TNa2Mn5O10+2AgCl ���С�ˮ������ں�ˮ�зŵ�ʱ���й�˵����ȷ���ǣ�������
| A�� | ������ӦʽAg+Cl--e-�TAgCl | |
| B�� | AgCl�ǻ�ԭ���� | |
| C�� | Na+������ˮ����صĸ����ƶ� | |
| D�� | ÿ����1 mol Na2Mn5O10ת��2 mol���� |