��Ŀ����
������һ�ַdz���Ҫ�Ļ���ԭ�ϣ��������������������������Ź㷺����;��
(1)����������ȡ��84����Һ��(��Ч�ɷ�ΪNaClO)��
�ٸ��Ʊ���Ӧ�����ӷ���ʽΪ ��
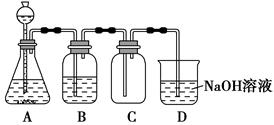
�����д�ʩ����ǿ��84����Һ��ɱ���������� ��
A��������������
B����������������
C����������NaOH��ĩ
�ۡ�84����Һ�����ܶԸ���(��Fe��C)��Ʒ����������ԭ���� ��
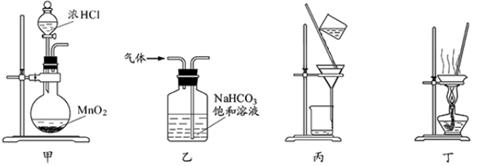
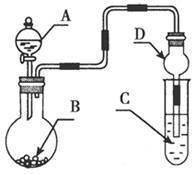
(2)��������(ClO2)��һ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������Ŀǰ����ҵ�Ͽ������õ�ⷨ��ȡClO2���¹��գ�����ԭ����ͼ��ʾ��
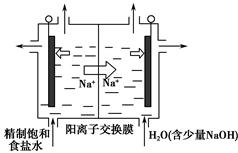
����ʾ��ͼ����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2��д����������ClO2�ĵ缫��Ӧʽ�� ��
(1)����������ȡ��84����Һ��(��Ч�ɷ�ΪNaClO)��
�ٸ��Ʊ���Ӧ�����ӷ���ʽΪ ��
�����д�ʩ����ǿ��84����Һ��ɱ���������� ��
A��������������
B����������������
C����������NaOH��ĩ
�ۡ�84����Һ�����ܶԸ���(��Fe��C)��Ʒ����������ԭ���� ��
(2)��������(ClO2)��һ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������Ŀǰ����ҵ�Ͽ������õ�ⷨ��ȡClO2���¹��գ�����ԭ����ͼ��ʾ��

����ʾ��ͼ����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2��д����������ClO2�ĵ缫��Ӧʽ�� ��
(1)��Cl2��2OH��=Cl����ClO����H2O
��A
�ۡ�84����Һ���Լ��ԣ������ڼ��������»ᷢ���绯ѧ��ʴ
(2)Cl����5e����2H2O=ClO2����4H��
��A
�ۡ�84����Һ���Լ��ԣ������ڼ��������»ᷢ���绯ѧ��ʴ
(2)Cl����5e����2H2O=ClO2����4H��
(1)��������NaOH��Һ��Ӧ���Ʊ���84����Һ�����ڼ����������ᣬ�ɴ�ʹClO��ת��ΪHClO����ǿ��84����Һ����ɱ���������������������ᣬH2SO3����ClO������������ԭ��Ӧ������ȷ���۸����ڼ����������ܷ���������ʴ�������á�84����Һ���Ը�����Ʒ����������(2)�ٵ�ⱥ��ʳ��ˮʱ������Cl������������Ӧ����ClO2������Ԫ���غ㣬��֪ˮ�����˷�Ӧ��������Ӧ��H����

��ϰ��ϵ�д�
�����Ŀ