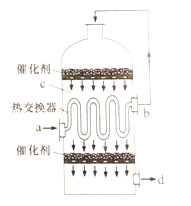
��Ŀ����
����Ŀ��(1)����������ȷ����____________________
A�����й��ۼ��Ļ�����һ���ǹ��ۻ�����
B�����ѷ��ֵ�����Ԫ�صĵ����ڳ��³�ѹ�¶�������
C������A��Ԫ�ص�ԭ�ӣ���뾶Խ��Խ���õ�����
D����n����Ԫ����������������ʽΪR2On���⻯�����ʽΪRHn (n��4)
E����������Ԫ����������ϼ۵�������������
F��ϡ������ԭ����ͬ����I A����A��Ԫ�صļ������Ӿ�����ͬ�ĺ�������Ų�
G�����ۻ������в����ܺ������Ӽ�
H��ֻ�зǽ���ԭ�Ӽ�����γɹ��ۼ�
I���ǽ���ԭ�Ӽ䲻�����γ����ӻ�����
(2)д���������ʵĵ���ʽ
MgBr2 ___________________ CCl4_______________HClO_______________
N2 ___________________ NH4Cl_______________NH3_______________
���𰸡�BG ![]()
![]()
![]()
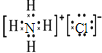
![]()
��������
��1��A�����й��ۼ��Ļ����ﲻһ���ǹ��ۻ����Ҳ���������ӻ������NaOH����A����
B��ϡ��������Ӽ�������С�����ѷ��ֵ�����Ԫ�صĵ����ڳ��³�ѹ�¶������壬��B��ȷ��
C���ڢ�A��Ԫ�ص�ԭ�ӣ���뾶Խ�˶Ե��ӵ�����ԽС��Խ�ѵõ����ӣ���C����
D����n����Ԫ��������������Ӧ�Ļ�ѧʽΪR2On����������ϼ�Ϊ+n�����������Ϊ-��8-n���������⻯�ﻯѧʽΪRH��8-n����n��4����H��8-n��R����D����
E����������Ԫ�أ��������=��������������Ԫ�ء���Ԫ��һ��û�������ϼۣ�����E����
F��ϡ������Heԭ����ͬ����H��������H+�ĺ�������Ų���ͬ����F����
G�����ݹ��ۻ�����Ķ��壬���ۻ������в����ܺ������Ӽ�����G��ȷ��
H�������ǽ���ԭ�Ӽ�Ҳ�����γɹ��ۼ�����AlCl3����H����
I���ǽ���ԭ�Ӽ�����γ����ӻ������NH4Cl����I����
��ѡBG��
��2��MgBr2Ϊ���ӻ������������Ҫ����������ӣ�![]() ��
��
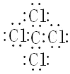
CCl4 �ǹ��ۻ����C���м䣬��Χ8�����ӣ�Clԭ�������ܣ�ÿ����ԭ����ΧҲ��8�����ӣ�ÿ��Clԭ�Ӷ���C�����������ӣ��������Ȼ�̼�ĵ���ʽ
HClOΪ���ۻ���������д���1���������1��Cl-O����������ĵ���ʽΪ��![]() ��
��
N2 ��Nԭ�������Ϊ5�����ӣ�����Nԭ�Ӽ��γ��������õ��Ӷԣ������ĵ���ʽΪ��![]() ��
��
NH4Cl�Ȼ��Ϊ���ӻ��������ʽ����Ҫ�����������������ɣ��Ȼ�淋ĵ���ʽΪ��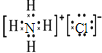 ��
��
NH3����Ϊ���ۻ���������д���3�����������ԭ�������ﵽ8�����ȶ��ṹ�������ĵ���ʽΪ��![]() ��
��

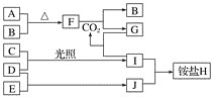
����Ŀ��CO2����Ҫ���������壬Ҳ��һ�ֹ�ҵԭ�ϡ���������CO2�����ڻ�������ЧӦ�����Ļ������⡣
(1)�ҹ���ѧ��ͨ������һ�������ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ������ֵ���͡�
��֪��2H2 (g)+O2 (g) =2H2O(l) ��H = -571.6 kJ/mol
2C8H18(l)+25O2(g) =16CO2(g)+18H2O(l) ��H = -11036 kJ/mol
25�桢101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)��Һ̬ˮ���Ȼ�ѧ����ʽ��_________��
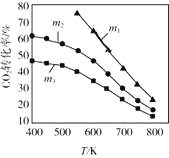
(2)CO2������ϳ��Ҵ��ķ�Ӧԭ���ǣ�2CO2(g)+6H2(g)![]() C2H5OH(g)+3H2O(g) ��H =-173.6 kJ/molͼ����ʼͶ�ϲ�ͬʱ��CO2��ƽ��ת�������¶ȵı仯��ϵ��mΪ��ʼʱ��Ͷ�ϱȣ���m=
C2H5OH(g)+3H2O(g) ��H =-173.6 kJ/molͼ����ʼͶ�ϲ�ͬʱ��CO2��ƽ��ת�������¶ȵı仯��ϵ��mΪ��ʼʱ��Ͷ�ϱȣ���m=![]() ��m1��m2��m3Ͷ�ϱȴӴ�С��˳��Ϊ_________��������_________��
��m1��m2��m3Ͷ�ϱȴӴ�С��˳��Ϊ_________��������_________��
(3)��Cu/ZnO���������£���CO2��H2��Ͽɺϳɼ״���ͬʱ������������ƽ�з�Ӧ��
��Ӧ�� CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H1=-53.7 kJ/mol��
CH3OH(g)+H2O(g) ��H1=-53.7 kJ/mol��
��Ӧ�� CO2(g)+H2(g) ![]() CO(g)+H2O(g)�� ����H2=+41.2 kJ/mol
CO(g)+H2O(g)�� ����H2=+41.2 kJ/mol
����һ����CO2��H2��ʼͶ�ϱȣ�����ͬѹǿ��,������ͬ��Ӧʱ��������ʵ������(�������״�ѡ��������ָת����CO2�����ɼ״��İٷֱ�)��
ʵ����� | T/K | ���� | CO2ת����/% | �״�ѡ����/% |
ʵ��1 | 543 | Cu/ZnO���װ� | 12.3 | 42.3 |
ʵ��2 | 543 | Cu/ZnO����Ƭ | 10.9 | 72.7 |
ʵ��3 | 553 | Cu/ZnO���װ� | 15.3 | 39.1 |
ʵ��4 | 553 | Cu/ZnO����Ƭ | 12.0 | 71.6 |
�ٶԱ�ʵ��1��ʵ��3�ɷ��֣�ͬ�����������£��¶����ߣ�CO2ת�������ߣ� ���״���ѡ����ȴ���ͣ�����ͼ״�ѡ���Խ��͵Ŀ���ԭ��_______________��
�ڶԱ�ʵ��1��ʵ�� 2�ɷ��֣���ͬ���¶��£�����Cu/ZnO����ƬʹCO2ת���ʽ��ͣ� ���״���ѡ����ȴ��ߣ�����ͼ״���ѡ������ߵĿ���ԭ��____________��
�����������CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��_______��
a��ʹ��Cu/ZnO���װ�������
b��ʹ��Cu/ZnO����Ƭ������
c�����ͷ�Ӧ�¶�
d��Ͷ�ϱȲ��䣬���ӷ�Ӧ���Ũ��
e������![]() �ij�ʼͶ�ϱ�
�ij�ʼͶ�ϱ�
(4)������������ĤΪ�����缫��ϡ����Ϊ�������Һ����һ��������ͨ��CO2����⣬���������Ƶõ��ܶȾ���ϩ![]() (���LDPE)��
(���LDPE)��
�ٵ��ʱ�������ĵ缫��Ӧʽ��_____________��
�ڹ�ҵ������1.4��104 kg ��LDPE����������Ҫ��״����______L ��CO2��
����Ŀ��1��2-���ȱ���(CH2C1CHClCH3)��һ����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͽ��ñ�ϩ�ӳɷ��Ʊ�����Ҫ������Ϊ3-�ȱ�ϩ(CH2=CHCH2C1)����Ӧԭ��Ϊ��
I��CH2=CHCH3(g)+C12(g)![]() CH2C1CHC1CH3(g) ��H1=��134kJ��mol-1
CH2C1CHC1CH3(g) ��H1=��134kJ��mol-1
II��CH2=CHCH3(g)+C12(g)![]() CH2=CHCH2C1(g)+HC1(g)��H2=��102kJ��mol-1
CH2=CHCH2C1(g)+HC1(g)��H2=��102kJ��mol-1
��ش��������⣺
(1)��֪CH2=CHCH2C1(g)+HC1(g)![]() CH2C1CHC1CH3(g)�Ļ��Ea(��)Ϊ132kJ��mol-1����÷�Ӧ�Ļ��Ea(��)Ϊ______kJ��mol-1��
CH2C1CHC1CH3(g)�Ļ��Ea(��)Ϊ132kJ��mol-1����÷�Ӧ�Ļ��Ea(��)Ϊ______kJ��mol-1��
(2)һ���¶��£�������ܱ������г�������ʵ�����CH2=CHCH3(g)��C12(g)���ڴ��������·�����ӦI���������������ѹǿ��ʱ��ı仯���±���ʾ��
ʱ��/min | 0 | 60 | 120 | 180 | 240 | 300 | 360 |
ѹǿ/kPa | 80 | 74.2 | 69.4 | 65.2 | 61.6 | 57.6 | 57.6 |
���õ�λʱ���������ѹ�ı仯����ʾ��Ӧ���ʣ���![]() ����ǰ120min��ƽ����Ӧ����v(CH2C1CHC1CH3)=______kPa��min-1��(����С�����2λ)��
����ǰ120min��ƽ����Ӧ����v(CH2C1CHC1CH3)=______kPa��min-1��(����С�����2λ)��
�ڸ��¶��£���ƽ��ʱHC1���������Ϊ![]() �����ϩ��ƽ����ת����
�����ϩ��ƽ����ת����![]() _______����ӦI��ƽ�ⳣ��Kp=_____kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��������С�����2λ)��
_______����ӦI��ƽ�ⳣ��Kp=_____kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ��������С�����2λ)��
(3)ij�о�С�����ܱ������г���һ������CH2=CHCH3��C12���ֱ���A��B���ֲ�ͬ���������·�����Ӧ��һ��ʱ�����CH2C1CHC1CH3�IJ������¶ȵĹ�ϵ����ͼ��ʾ��
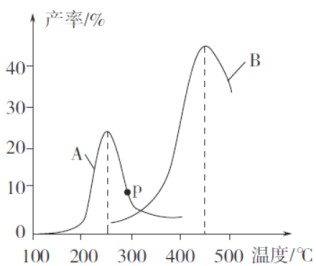
������˵���������___________(�����)��
a��ʹ�ô���A������¶�ԼΪ250��
b����ͬ�����£��ı�ѹǿ��Ӱ��CH2C1CHC1CH3�IJ���
c�����ִ������ܽ��ͷ�Ӧ�Ļ�ܣ�����H����
d�����CH2C1CHC1CH3��Ӧѡ���ԵĹؼ������ǿ����¶�
���ڴ���A�����£��¶ȵ���200��ʱ��CH2C1CHC1CH3�IJ������¶����߱仯������Ҫԭ����_______________________________________________________________��
��p���Ƿ�Ϊ��Ӧ�¶���CH2C1CHC1CH3��ƽ����ʣ��ж�������_____________��