��Ŀ����
����������CO2���ڻ��ۣ���в����̬����������������ܵ�ȫ�����ע��
��1����ҵ�ϳ��ø�Ũ�ȵ�K2CO3��Һ����CO2������ҺX�������õ�ⷨʹK2CO3��Һ��������װ��ʾ��ͼ���£�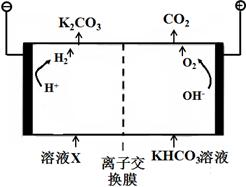
�������������ķ�Ӧ���� ��H ++ HCO3-=H2O+CO2����
����CO32-��������������ԭ�� ��
��2������װ���в�����CO2��H2��һ�������·�Ӧ���ɼ״��Ȳ����ҵ�����ø÷�Ӧ�ϳɼ״���
��֪��25 �棬101 KPa�£�
H2(g)+1/2 O2(g)=H2O(g) �� H1=" -242" kJ/mol
CH3OH(g)+3/2 O2(g)=CO2 (g)+2 H2O(g) �� H2=" -676" kJ/mol
д��CO2��H2������̬�״��Ȳ�����Ȼ�ѧ����ʽ ��
�����ʾ�ϳɼ״��ķ�Ӧ�������仯ʾ��ͼ��������ȷ���� ������ĸ��ţ���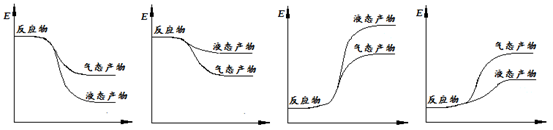
a b c d
��3������ȼ�ϵ����һ���������ォ��ѧ��ֱ��ת���ɵ��ܵ�װ�á���֪ij�ּ״�
����ȼ�ϵ���У��������ҺΪ���ԣ�ʾ��ͼ���£�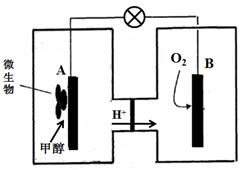
�õ�����·���ӵ���������Ϊ ����д����A��B����B��A������
����������B�缫����Һ��pH�빤��ǰ��Ƚ� ����д����������С�����䡱����Һ����仯���Բ��ƣ���
A�缫�����״������ĵ缫��ӦʽΪ ��
��1����4OH--4e-=2H2O+O2����2�֣�
�ڴ�1��HCO3 �C���ڵ���ƽ�⣺HCO3 �C H++CO32- ��1�֣�������H+�ŵ�Ũ�ȼ�Сƽ�����ƣ�1�֣�CO32-����
H++CO32- ��1�֣�������H+�ŵ�Ũ�ȼ�Сƽ�����ƣ�1�֣�CO32-����
��2������H+�ŵ�OH-Ũ������1�֣���OH-��HCO3 �C��Ӧ����CO32-��1�֣�CO32-����
��2����CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H="-50" kJ/mol��2�֣� �� a��2�֣�
��3���ٴ�A��B��1�֣� �ڲ��䣨1�֣�
��CH3OH+H2O - 6e-=6H++ CO2����2�֣�
���������������1����������H2O�������OH?ʧ���ӣ��缫����ʽΪ��4OH - - 4e-=2H2O+O2��
��������H+�ŵ緢���õ��ӷ�Ӧ:2H++2e?=H2��,HCO3?���ڵ���ƽ�⣺HCO3? CO32?+H+��H+Ũ�ȼ�С��ʹHCO3?����ƽ�������ƶ���CO32?Ũ�������������
CO32?+H+��H+Ũ�ȼ�С��ʹHCO3?����ƽ�������ƶ���CO32?Ũ�������������
��2��������д��CO2��H2��Ӧ���ɼ״��Ļ�ѧ����ʽ����ע��״̬��Ȼ����ݸ�˹������?H=3?H1��?H2= -50 kJ?mol?1�������Ȼ�ѧ����ʽΪ��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H="-50" kJ?mol?1��
�ڸ÷�ӦΪ���ȷ�Ӧ��������ΪҺ̬���ų����������࣬����a��ͼ����ȷ��
��3�� �ټ״�ʧȥ���ӣ�Ϊ��صĸ��������Ըõ�����·���ӵ���������Ϊ��A��B��
��B�缫��O2�õ�������H+��ͬʱ��Һ�е�H+����B�缫�ң�����B�缫����Һ��pH�빤��ǰ��Ƚ����䡣
��CH3OHʧ���ӣ�����CO2��H+�����ݻ��ϼ۱仯��Ԫ���غ���ƽ����ʽ��CH3OH+H2O - 6e-=6H++ CO2��
���㣺���⿼����ԭ����ԭ���ԭ��������ʽ����д��ƽ���ƶ����Ȼ�ѧ����ʽ����д��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� O2(g)=CO2(g)�� ��H2��b kJ��mol��1
O2(g)=CO2(g)�� ��H2��b kJ��mol��1 2NH3(g)����H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol(�����1��1)����5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������
2NH3(g)����H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol(�����1��1)����5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
 CH3OH(g) ��H <0��
CH3OH(g) ��H <0��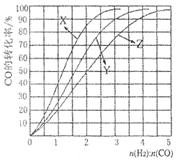
 O2��g����H2 O��g�� ��H����241.8kJ��mol��1
O2��g����H2 O��g�� ��H����241.8kJ��mol��1 2NO��g�� ��H
2NO��g�� ��H 0����1.0 mol������0.80 mol N2��0.20 mol O2��1300oCʱ��1.0 L�ܱ������ھ���5s��Ӧ�ﵽƽ�⣬���NOΪ8.0��10��4 mol��
0����1.0 mol������0.80 mol N2��0.20 mol O2��1300oCʱ��1.0 L�ܱ������ھ���5s��Ӧ�ﵽƽ�⣬���NOΪ8.0��10��4 mol�� 2CO2��g����N2��g�� �У�NO��Ũ��
2CO2��g����N2��g�� �У�NO��Ũ��
 2N (g)
2N (g) 2H (g)
2H (g)
 ��3������һ��DZ�ڵ������Դ������������ȼ�ϵ�ص�ȼ�ϡ���ص��ܷ�ӦΪ��
��3������һ��DZ�ڵ������Դ������������ȼ�ϵ�ص�ȼ�ϡ���ص��ܷ�ӦΪ��


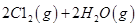

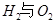 ��Ӧ������̬ˮ���Ȼ�ѧ����ʽ__________________________
��Ӧ������̬ˮ���Ȼ�ѧ����ʽ__________________________

 =0��5����400��ʱ����0��5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ
=0��5����400��ʱ����0��5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��Ӧ ____________
____________ �������������������ȷ������
�������������������ȷ������
 ���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
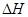 ___________0�������������
___________0�������������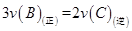 B��������ѹǿ���ֲ���
B��������ѹǿ���ֲ��� CH3OH(g)+H2O(g)��ͼ1��ʾ�÷�Ӧ����������(��λΪkJ��mol��1)�ı仯��
CH3OH(g)+H2O(g)��ͼ1��ʾ�÷�Ӧ����������(��λΪkJ��mol��1)�ı仯��
 ������� ��
������� ��