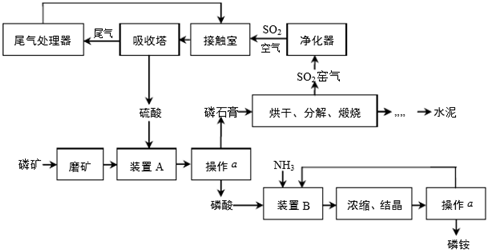
��Ŀ����
13��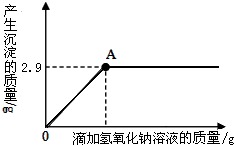 ��һ�����İ�ɫ�����Ȼ�þ�к��������Ȼ��ƣ���ѧ��ȤС���ͬѧΪ�˲ⶨ�ù����Ȼ�þ���Ȼ��Ƶ���������������������ʵ�飺����10g��Ʒ�����ձ��У������м���90gˮ�����裬ʹ����ȫ�ܽ�Ϊֹ��Ȼ��ȡ����Һһ�룬�����еμ�10%������������Һ����������������������������������Һ��������ϵ������ͼ��ʾ��
��һ�����İ�ɫ�����Ȼ�þ�к��������Ȼ��ƣ���ѧ��ȤС���ͬѧΪ�˲ⶨ�ù����Ȼ�þ���Ȼ��Ƶ���������������������ʵ�飺����10g��Ʒ�����ձ��У������м���90gˮ�����裬ʹ����ȫ�ܽ�Ϊֹ��Ȼ��ȡ����Һһ�룬�����еμ�10%������������Һ����������������������������������Һ��������ϵ������ͼ��ʾ���Լ��㣺������������С����1λ��
��1����Ʒ���Ȼ�þ��������
��2��������10%������������Һ��A��ʱ��������Һ���Ȼ��Ƶ�����������
���� ��1���������⡰����10g��Ʒ�����ձ��У������м���90gˮ�����裬ʹ����ȫ�ܽ�Ϊֹ��Ȼ��ȡ����Һһ�롱��ͼ����Ϣ����֪����5g��Ʒ����ˮ��Ӧ���ɳ���������Ϊ2.9g���ó���Ϊ������þ������������þ��������Ϸ�Ӧ�Ļ�ѧ����ʽ����������Ȼ�þ������������������Ȼ��Ƶ�������������������
��2����Ӧ���Ȼ�����Һ��������Դ����Ʒ�к��еĺͷ�Ӧ�����ɵģ���Ӧ����Һ���������Ը��������غ㶨�������㣬����Ӧ����Һ������=��Ӧǰ����ݵ�����֮��-���������-�����������ʣ���������
��� �⣺��������ͼ�ɵ�Mg��OH��2������Ϊ2.9 g��
��MgCl2����Ϊx���μӷ�Ӧ��NaOH������Ϊy�����ɵ�NaCl������Ϊz����
MgCl2+2NaOH�T2NaCl+Mg��OH��2��
95 80 117 58
x y��10% z 2.9g
95��58=x��2.9g
x=95��2.9��58=4.75 g
��Ʒ��MgCl������Ϊ4.75��2=9.5 g��
��Ʒ��NaCl������Ϊ��10-4.75��2��=0.5 g��
80��58=y��10%��2.9��
y=80��2.9��5.8=40g
117��58=Z��2.9g
z=117��2.9��58
=5.85 g
��Һ��NaCl��������=5.85g+0.25g=6.1g
A��ʱ��������Һ��NaCl����������=6.1g�£�50+40-2.9����100%=7%��
����Ʒ���Ȼ�þ������Ϊ9.5 g��������10%������������Һ��A��ʱ��������Һ���Ȼ��Ƶ���������Ϊ7%��
���� ���⿼������ļ��㣬������ѧ���ķ��������������Ŀ��飬ע��������غ�ĽǶȽ����⣬�Ѷ��еȣ�

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�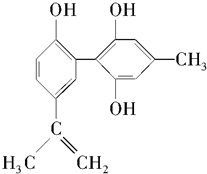
| A�� | ��FeCl3��Һ����ɫ����Ϊ�������뱽������ͬϵ�� | |
| B�� | �÷����в���������̼ԭ�ӹ�ƽ�� | |
| C�� | ����������Ũ��ˮ�����ӳɷ�Ӧ��ȡ����Ӧ | |
| D�� | 1 mol�������ڴ����������������7 molH2�����ӳɷ�Ӧ |
| A�� | I2���� | B�� | �ռ�����ˮ | C�� | HCl����ˮ������ | D�� | ������������ˮ |
| A�� | �ⵥ�� | B�� | ��ԭ�� | C�� | ��Ԫ�� | D�� | ������ |
| A�� | ��ʳ�׳�ȥůƿ�ڵ�ˮ�� | B�� | ��������Һ�������� | ||
| C�� | �ⵥ�������۱��� | D�� | ������ϴȥ�����ϵ����� |
| A�� | ������ζ��������������ʳ�� | |
| B�� | �������ô���ˮ���������彡�� | |
| C�� | ����ʳ�û����ͱ����������ڽ��� | |
| D�� | ����Ȼ���ʶ�����ɫ�������� |
| A�� | ����غͽ��ʯ | B�� | ������ˮ�� | C�� | ���ɱ� | D�� | �Ȼ��ƺ����� |