��Ŀ����
8��Ư���dz��õ�������������Ҫ�ɷ����Ȼ��ƣ�CaCl2���ʹ������[Ca��ClO��2]�����ǰ�ɫ��ĩ������ǿ������ζ������ˮ����ѧ���ʲ��ȶ������л�������ȿ�����ȼ�գ�������֯Ʒ��ֽ����Ư�ס���ˮ�������߲˹Ϲ�������ˮ���������������ȣ���������ҩ����ɫ�ȣ���1����������������ѡ��л�����������
��2���������ݹ��ɳ�Ư�۵��������ʰ�ɫ��ĩ������ǿ������ζ������ˮ��
��3����ҵ�Ͻ�����ͨ��ʯ����[Ca��OH��2]��ȡƯ�ۣ�д���÷�Ӧ�Ļ�ѧ����ʽ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
���� ��1���������[Ca��ClO��2]������CԪ�أ�
��2������Ϣ��֪�����ʵ�״̬����ɫ����ζ���ܽ��Ե��������ʣ�
��3������ͨ��ʯ����[Ca��OH��2]��ȡƯ�ۣ���Ӧ�����Ȼ��ơ�������ƺ�ˮ��
��� �⣺��1���������[Ca��ClO��2]������CԪ�أ���������ʴ�Ϊ�����
��2������Ϣ��֪���������[Ca��ClO��2]�����ǰ�ɫ��ĩ������ǿ������ζ������ˮ������������Ϊ��ɫ��ĩ������ǿ������ζ������ˮ��
�ʴ�Ϊ����ɫ��ĩ������ǿ������ζ������ˮ��
��3������ͨ��ʯ����[Ca��OH��2]��ȡƯ�ۣ���Ӧ�����Ȼ��ơ�������ƺ�ˮ����ӦΪ2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
�ʴ�Ϊ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
���� ���⿼��Ư�ۼ��Ʊ���Ϊ��Ƶ���㣬����ϰ���е���Ϣ���Ʊ�Ư�۵�ԭ��Ϊ���Ĺؼ���ע��Ǩ��Ӧ����������Ϣ���������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
18��1919�꣬��ѧ�ҵ�һ��ʵ����������������--�˹�ת��Ԫ�أ�����˷�Ӧ���£�${\;}_{7}^{14}$N+${\;}_{2}^{4}$He��${\;}_{8}^{17}$O+${\;}_{1}^{1}$H����������ȷ���ǣ�������
| A�� | ${\;}_{8}^{17}$Oԭ�Ӻ�����9������ | B�� | Hԭ�Ӻ�����1������ | ||
| C�� | O2��O3��Ϊͬλ�� | D�� | 32He��42He�����ֲ�ͬ�ĺ��� |
16���淶ʵ������ǻ��ʵ��ɹ�����Ҫ��֤������ʵ�����������ǣ�������
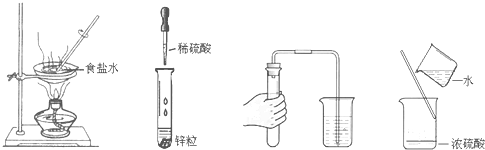

| A�� | ����ʳ��ˮ | B�� | �ιܵ�ʹ�� | C�� | ���װ�������� | D�� | ϡ��Ũ���� |
3�������£�X��Y��Z�������ʵĽ���pH��ͼ��ʾ������˵��������ǣ�������
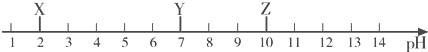

| A�� | Xһ����ϡ���� | B�� | ��̪��Һ��Z��Һ���ɫ | ||
| C�� | Y��Һ��������Һ | D�� | X��Һ��ʹʯ����Һ��� |
20����NAΪ����٤��������ֵ������˵����ȷ���ǣ�������
| A�� | 10mL 18mol/LŨ����������ͭ���ȳ�ַ�Ӧ��ת�Ƶ�����Ϊ0.18NA | |
| B�� | 0.1mol24Mg32S������������������Ϊ2.8NA | |
| C�� | ��״���£�22.4LCO2�к��еĹ�ͬ���Ӷ���Ϊ2NA | |
| D�� | 6.0g���ᾧ���к��е�H+��Ϊ0.1NA |
17��1L 0.2mol/L��KI��Һ�У�������KI�����ʵ����ǣ�������
| A�� | 0.2mol | B�� | 0.5mol | C�� | 2mol | D�� | 5mol |
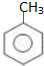
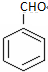
18�������ºϳ�·�ߣ�������ת��Ϊ����������������ȷ���ǣ�������
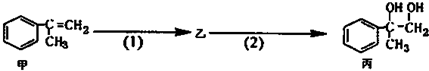
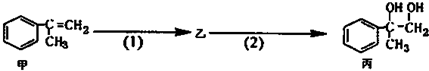
| A�� | ���п��ܺ���δ��Ӧ�ļף�������ˮ�����Ƿ� | |
| B�� | ��Ӧ��1�������Լ���Һ�壬�������� | |
| C�� | �ͱ�����������KMnO4��Һ������Ӧ | |
| D�� | ��Ӧ��2����Ӧ����ȡ����Ӧ |
 ��
�� �ĺ˴Ź�������ͼ����4��壮
�ĺ˴Ź�������ͼ����4��壮 +CH3COOH$��_{��}^{Ũ����}$
+CH3COOH$��_{��}^{Ũ����}$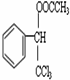 +H2O��
+H2O��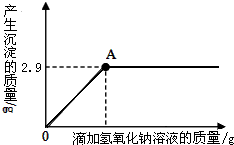 ��һ�����İ�ɫ�����Ȼ�þ�к��������Ȼ��ƣ���ѧ��ȤС���ͬѧΪ�˲ⶨ�ù����Ȼ�þ���Ȼ��Ƶ���������������������ʵ�飺����10g��Ʒ�����ձ��У������м���90gˮ�����裬ʹ����ȫ�ܽ�Ϊֹ��Ȼ��ȡ����Һһ�룬�����еμ�10%������������Һ����������������������������������Һ��������ϵ������ͼ��ʾ��
��һ�����İ�ɫ�����Ȼ�þ�к��������Ȼ��ƣ���ѧ��ȤС���ͬѧΪ�˲ⶨ�ù����Ȼ�þ���Ȼ��Ƶ���������������������ʵ�飺����10g��Ʒ�����ձ��У������м���90gˮ�����裬ʹ����ȫ�ܽ�Ϊֹ��Ȼ��ȡ����Һһ�룬�����еμ�10%������������Һ����������������������������������Һ��������ϵ������ͼ��ʾ��