��Ŀ����
ijѧϰС��Ϊ֤�����۲�ͭ��ϡHNO3��Ӧ�IJ�����NO�����������ͼ��ʾ��ʵ��װ�á�����������ǵ�˼·�����������ʵ�鱨�档
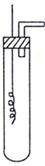
��1��ʵ���������Թܡ����ܡ��ձ�������ע������
��2��ʵ��ҩƷ��ͭ˿��ϡ���ᡢ�ռ���Һ�����ۡ�CaCO3������
��3��ʵ��ԭ����
ͭ��ϡ��������ӷ�Ӧ����ʽ ��
��4��ʵ�鲽�裺
��ʵ�鿪ʼʱ��������еĵ�һ�������Ǽ���װ�õ� ��
�����Թ��м���һ�����Ĺ���ҩƷ ��Ȼ�����Թ��е��������ϡ���ᣬ��Ѹ��������ͭ˿�͵��ܵ���Ƥ����
�۷�Ӧһ��ʱ�����պ��NaOH��Һ�����ŷ�ס���ܿڣ�
�ܽ�ͭ˿�����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ��
�ݽ�ע���������Թܿڵ���Ƥ���У����Թ������������
��5��ʵ�����ۣ�
��ʵ�鲽��ڵ�Ŀ���� ��
��ʵ�鲽��۵�Ŀ���� ��
��ʵ�鲽��ݹ۲쵽�������� ��
��6��ʵ�����ۣ�
��װ�õ��ŵ��ǣ�ֻ��һ���ŵ㼴�ɣ� ��

��1��ʵ���������Թܡ����ܡ��ձ�������ע������
��2��ʵ��ҩƷ��ͭ˿��ϡ���ᡢ�ռ���Һ�����ۡ�CaCO3������
��3��ʵ��ԭ����
ͭ��ϡ��������ӷ�Ӧ����ʽ ��
��4��ʵ�鲽�裺
��ʵ�鿪ʼʱ��������еĵ�һ�������Ǽ���װ�õ� ��
�����Թ��м���һ�����Ĺ���ҩƷ ��Ȼ�����Թ��е��������ϡ���ᣬ��Ѹ��������ͭ˿�͵��ܵ���Ƥ����
�۷�Ӧһ��ʱ�����պ��NaOH��Һ�����ŷ�ס���ܿڣ�
�ܽ�ͭ˿�����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ��
�ݽ�ע���������Թܿڵ���Ƥ���У����Թ������������
��5��ʵ�����ۣ�
��ʵ�鲽��ڵ�Ŀ���� ��
��ʵ�鲽��۵�Ŀ���� ��
��ʵ�鲽��ݹ۲쵽�������� ��
��6��ʵ�����ۣ�
��װ�õ��ŵ��ǣ�ֻ��һ���ŵ㼴�ɣ� ��
��12�֣���3��3Cu+8H+ + 2NO3����3Cu2+ + 2NO��+ 4H2O��2�֣�
��4���������ԣ���1�֣���CaCO3������1�֣�
��5�����ų��Թ��еĿ�������ֹ��NO��Ӧ��2�֣�����ʹ��NaOH��Һ���ղ�����NO��NO2���壬��ֹ�ݳ���Ⱦ������2�֣�������������ɫ�����ɫ��2�֣�
��6��ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ�������2�֣�����ʹ��պ��NaOH��Һ�����ŷ�ס���ܿڣ��ɷ�ֹNO��NO2�����ݳ���Ⱦ��������
��4���������ԣ���1�֣���CaCO3������1�֣�
��5�����ų��Թ��еĿ�������ֹ��NO��Ӧ��2�֣�����ʹ��NaOH��Һ���ղ�����NO��NO2���壬��ֹ�ݳ���Ⱦ������2�֣�������������ɫ�����ɫ��2�֣�
��6��ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ�������2�֣�����ʹ��պ��NaOH��Һ�����ŷ�ס���ܿڣ��ɷ�ֹNO��NO2�����ݳ���Ⱦ��������
�����������3���������������ᣬ��ͭ��Ӧ��������ͭ��NO��ˮ����ͭ��ϡ��������ӷ�Ӧ����ʽ��3Cu+8H+ + 2NO3����3Cu2+ + 2NO��+ 4H2O��
��4����װ�����Ӻ��Ժ������װ�õ������ԣ����ʵ�鿪ʼʱ��������еĵ�һ�������Ǽ���װ�õ������ԡ�
������ͭ��ϡ���ᷴӦ����NO���ױ���������NO2����װ���к��п�����������ų�װ���еĿ��������Կ���̼��ƺ����ᷴӦ���ɵ�CO2��ʵ�֡�
��5����ʵ�鲽��ڵ�Ŀ�����ų��Թ��еĿ�������ֹ��NO��Ӧ��
�����ڵ����������Ǵ�����Ⱦ�����ʵ�鲽��۵�Ŀ����ʹ��NaOH��Һ���ղ�����NO��NO2���壬��ֹ�ݳ���Ⱦ������
��NO���ױ������������ɺ���ɫNO2������ʵ�鲽��ݹ۲쵽����������������ɫ�����ɫ��
��6������װ��ͼ��֪����װ�õ��ŵ���ʹ�ÿɳ鶯��ͭ˿����ʱ���Ʒ�Ӧ�Ŀ�ʼ�ͽ�������ԼҩƷ������������Ⱦ����IJ�������ʹ��պ��NaOH��Һ�����ŷ�ס���ܿڣ��ɷ�ֹNO��NO2�����ݳ���Ⱦ��������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����������У������ǿ��ּ����������淶�Ͻ���ʵ����������Ͷ��ֲ�����������������������Ļ���������ʶ�����������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

��ϰ��ϵ�д�
�����Ŀ
 ��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�
��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�

