��Ŀ����
��13�֣���ѧ�ڻ�������������ʮ����Ҫ�����á������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
��1�������������У�H2�ܽ�NO ��ԭΪN2��25��ʱ����Ӧ����10 min����Һ��pH��7��Ϊ12�������£���
��ԭΪN2��25��ʱ����Ӧ����10 min����Һ��pH��7��Ϊ12�������£���
�� ��֪��pH=-lgc(H+),�����£�ˮ��Һ��c(H+)��c(OH-)=10-14 ��
��N2�ĽṹʽΪ________��
���벹�����ӷ���ʽ����������ƽ��������Ӧ���ӷ���ʽΪ(��ƽΪ1��ʡ��)
�� ��NO ���� ��H2���� �� ��N2���� ��H2O���� ��__________��
���� ��H2���� �� ��N2���� ��H2O���� ��__________��
��ƽ����Ӧ����v(NO )= mol��L��1��min��1��
)= mol��L��1��min��1��
��2���绯ѧ����NO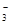 ��ԭ����ͼ��ʾ��
��ԭ����ͼ��ʾ��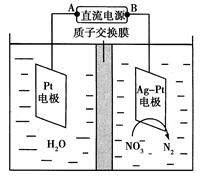
�ٵ�Դ����Ϊ________(�A����B��)��
������ӦʽΪ ��
������������ת����2 mol���ӣ���Ĥ������Һ�������仯��(��m������m��)Ϊ________g��
��1����N��N ��2�֣�
��2NO3?+5H2 N2+2OH?+4H2O ��3�֣���ƽȫ�Ե�2�֣�OH?�Ե�1�֣� 0.001 ��2�֣�
N2+2OH?+4H2O ��3�֣���ƽȫ�Ե�2�֣�OH?�Ե�1�֣� 0.001 ��2�֣�
��2���� A ��2�֣�2NO3?+6H2O+10e?=N2��+12OH? ��3�֣�
��14.4 ��3�֣�
���������������1����N2������Nԭ�Ӽ��γ��˵������������ԽṹʽΪ��N��N��
�ڸ���ԭ���غ�͵���غ㣬�����ﻹ��OH?�����ݻ��ϼ۱仯��NԪ����+5�۽���Ϊ0�ۣ���N2Ϊ�о���������10�ۣ�H2��HԪ����0��������+1�ۣ�������2�ۣ����ݻ��ϼ����ߺͽ��͵ļ�����ȣ���ƽ�ɵ����ӷ���ʽ��2NO3?+5H2 N2+2OH?+4H2O������pH=-lgc(H+)��pH��7��Ϊ12������Һ��H+Ũ����10-7��Ϊ10-12������ˮ��Һ��c(H+)��c(OH-)=10-14 ���ɵ�?c��OH?��=0.01mol?L?1����v(NO)=0.01mol?L?1��10min=0.001 mol��L��1��min��1��
N2+2OH?+4H2O������pH=-lgc(H+)��pH��7��Ϊ12������Һ��H+Ũ����10-7��Ϊ10-12������ˮ��Һ��c(H+)��c(OH-)=10-14 ���ɵ�?c��OH?��=0.01mol?L?1����v(NO)=0.01mol?L?1��10min=0.001 mol��L��1��min��1��
��2���ٵ��ص��Ҳ�NO3?ת��ΪN2��NԪ�صõ��ӷ�����ԭ��Ӧ����Ag��PtΪ���ص�������BΪ��Դ�ĸ��������Ե�Դ����ΪA����������NO3?�õ�������N2�����ݻ��ϼۺ͵���ת�ơ�ԭ���غ㡢����غ�ɵõ缫��ӦʽΪ��2NO3?+6H2O+10e?=N2��+12OH?
�ڵ��ص�������Ӧ:2H2O��4e?=4H++O2��������������ת����2 mol���ӣ��ų�0.5mol O2��2mol H+ͨ�����ӽ���Ĥ�����Ҳ࣬���������Һ�������٣�0.5mol��32g/mol+2mol��1g/mol=18g�����ص��Ҳ����Ӧ��2NO3?+6H2O+10e?=N2��+12OH?������������ת����2 mol���ӣ��ų�0.2mol N2����������2g H+�������Ҳ�������Һ�������٣�0.2mol��28g/mol��2g=3.6g����Ĥ������Һ�������仯��(��m������m��)Ϊ��18g��3.6g=14.4g��
���㣺���⿼�����ʵĽṹ�����ʡ�����ʽ����д�����ԭ������ؼ��㡣

 ��������������������ϵ�д�
��������������������ϵ�д������������ʵıȽϣ���ȷ����
| A�����ȶ��ԣ�Na2CO3>NaHCO3>H2CO3 |
| B���۵㣺K>Na>Li |
| C��ͬ�����£�ͬŨ����Һ��pHֵ��NaHCO3>Na2CO3 |
| D����ԭ�ԣ�S2->Br->I- |
������NO�ķ�Ӧԭ��Ϊ��2CO��2NO=N2��2CO2�йظ÷�Ӧ��˵������ȷ���� ( )
| A����Ӧ��COΪ������ |
| B����Ӧ��NO����ԭ |
| C���ڷ�Ӧ����1 mol N2ʱ��ת�Ƶĵ���Ϊ4 mol |
| D��CO��NO������ɫ�ж����� |
(10��)Ϊ�˷�ֹǹ֧���⣬����ǹ֧�ĸ����������NaNO2��NaOH�Ļ��Һ�н��л�ѧ����ʹ���������������Fe3O4�����ܵı����㡪����������������̿������л�ѧ����ʽ��ʾ�� �� 3Fe��NaNO2��5NaOH��3Na2FeO2��H2O��NH3��
�� ��Na2FeO2����NaNO2����H2O ������Na2Fe2O4����NH3������NaOH
�� Na2FeO2��Na2Fe2O4��2H2O Fe3O4��4NaOH
Fe3O4��4NaOH
��1����ƽ��ѧ����ʽ�ڡ�
��2��������Ӧ���л�ԭ��Ϊ ������1mol Na2FeO2���ɣ���Ӧ�������� mol����ת�ơ�
��3�������γɡ��������Ĺ��̣�����˵����ȷ���� ��
| A�����������̲��������Ⱦ | B����Ӧ�����ɵ��������������п���ʴ���� |
| C����Ӧ�٢ڢ۾���������ԭ��Ӧ | D����Ӧ�٢��е���������ΪNaNO2 |
���������������������������������������������������������������� ��
Ϊ֤��Fe3+���н�ǿ�������ԣ���ͬѧ��������ʵ�飺��CuƬ����0.5mol/L Fe(NO3)3��Һ�У��۲쵽CuƬ���ܽ⣬��Һ�ɻ�ɫ��Ϊ����ɫ���ɴ˼�ͬѧ�õ�Fe3+���н�ǿ�����ԵĽ��ۡ�
��ͬѧ����˲�ͬ�Ŀ�������Fe(NO3)3��Һ�������ԣ��ڴ�����������NO3-Ҳ������Cu���������ʵ�����̽������֪��
| ˮ�ⷴӦ | ƽ�ⳣ����K�� |
Fe3+ + 3H2O  Fe(OH)3 + 3H+ Fe(OH)3 + 3H+ | 7.9 �� 10-4 |
Fe2+ + 2H2O  Fe(OH)2 + 2H+ Fe(OH)2 + 2H+ | 3.2 �� 10-10 |
Cu2+ + 2H2O  Cu(OH)2 + 2H+ Cu(OH)2 + 2H+ | 3.2 �� 10-7 |
��ش𣺣�1��ϡ�����Cu��Ӧ�Ļ�ѧ����ʽΪ ��
��2�����������ṩ���Լ���������ͬѧ���ʵ�鷽����ơ�
�Լ���0.5mol/L Fe(NO3)3��Һ��CuƬ������pH��ֽ��0.5��5.0������������Һ��ϡ���ᡣ
������ ��
��3����ͬѧ�ֱ�ʵʩ�˼ס�����λͬѧ��ʵ�鷽��������ʵ���������pH�Ƽ����ҺpH�ı仯��ʵ���¼���¡�
| ʵ������ | ʵ������ |
| ��ͬѧ��ʵ�鷽�� | ��Һ�������ɫ�� pH�������� |
| ��ͬѧ��ʵ�鷽�� | ����������pHû�����Ա仯�� |
�پ�ʵ������д��������Ӧ�����ӷ���ʽ�� ��
�ڵ���ʵ���������ҺpH���������Ŀ���ԭ���� ��
�۽�����ͬѧ��ʵ������ ��
��4��������Ƹ������е�ʵ�鷽����������ͬѧ�ﵽʵ��Ŀ�ģ� ��
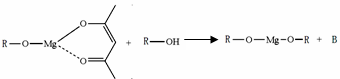
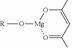 �����ɷ������·�Ӧ��
�����ɷ������·�Ӧ��