��Ŀ����
����Ŀ�������£��� 0.1mol/LNa2A��Һ�в���ͨ��HCl��H2A��HA-��A2-����Һ����ռ���ʵ��������� pOH[pOH=-lgc(OH-)]�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
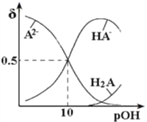
A. H2A�ĵڶ�������ƽ�ⳣ��Ϊ10-10
B. ����HCl��ͨ�� c(H+)/c(H2A)�ȼ�С������
C. ����Һ������ʱ:c(Na+)=c(HA-)+2c(A2-)
D. ����Ũ�ȵ������ Na2A��H2A��Һ��Ϻ���Һ�Լ���
���𰸡�B
��������A����pOH=10ʱ��c��H+��=l0-4mol/L��HA-��A2-Ũ����ȣ�H2A�ĵڶ�������ƽ�ⳣ��Ϊ![]() ��ѡ��A����B���¶Ȳ��䣬��H2A�ĵ�һ�������ƻ��������䣬��
��ѡ��A����B���¶Ȳ��䣬��H2A�ĵ�һ�������ƻ��������䣬��![]() ���䣬����HA-Ũ���������С����
���䣬����HA-Ũ���������С����![]() �ȼ�С������ѡ��B��ȷ��C������Һ������ʱ��c��Na+���Tc��HA-��+2c ��A2-��+c��Cl-����ѡ��C����
�ȼ�С������ѡ��B��ȷ��C������Һ������ʱ��c��Na+���Tc��HA-��+2c ��A2-��+c��Cl-����ѡ��C����
D������Ũ�ȵ������Na2A��H2A��Һ��Ϻ�Ӧ����NaHA����A��֪H2A�ĵڶ�������ƽ�ⳣ��Ϊ![]() ����HA-��ˮ�ⳣ��Ϊ
����HA-��ˮ�ⳣ��Ϊ![]() ����֪HA-�������ˮ��̶ȣ���Һ�����ԣ�ѡ��D����ѡB��
����֪HA-�������ˮ��̶ȣ���Һ�����ԣ�ѡ��D����ѡB��

��ϰ��ϵ�д�
�����Ŀ
