��Ŀ����
1��Ϊ�˼���CO�Դ�������Ⱦ��ij�о���ѧϰС�����о�����CO��H2O��Ӧת��Ϊ��ɫ��ԴH2����֪��2CO��g��+O2��g���T2CO2��g����H=-566.0kJ•moL-1
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ•moL-1
H2O��g���TH2O��l����H=-44.0kJ•moL-1
��1��д��CO��H2O��g����������CO2��H2���Ȼ�ѧ����ʽ��CO��g��+H2O��g��=CO2��g��+H2��g����H=-41.2kJ•mol-1
��2�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�ѧ����ʽ���£�
N2��g��+3H2��g��?2NH3��g����H=-92.4kJ•moL-1
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ������������ı�N2��H2��NH3����������Ӧ������ʱ��Ĺ�ϵ��ͼ1��ʾ��

ͼ��t3ʱ����ƽ���ƶ������������������¶ȣ�
���б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����t2-t3ʱ�Σ�
���¶�ΪT��ʱ����1mol N2��2mol H2�����ݻ�Ϊ0.5L���ܱ������У���ַ�Ӧ����N2��ƽ��ת����Ϊ50%����Ӧ��T��ʱ��ƽ�ⳣ��Ϊ16mol-2•L2��
��Ŀǰ��ҵ�ϳɰ���ԭ���ǣ�
N2+3H2 $?_{500�桢����ý}^{20-50MPa}$2NH3
��ͼ2��ʾ�������ı䣬ƽ����ϵ�а�����������ı仯���ƣ���������Ϊѹǿʱ���仯������ȷ���ǣ�����ţ���ͬ��c����������Ϊ�¶�ʱ���仯������ȷ����a��
��3�������°�����HCl����������ˮ���ֽ���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������ϣ�������Һ�и����ӵ����ʵ���Ũ�Ȱ����ɴ�С��˳����������Ϊc��Cl-����c��NH4+����c��H+����c��OH-����
���� ��1����֪���٣�2CO��g��+O2��g���T2CO2��g����H=-566.0kJ•moL-1
��.2H2��g��+O2��g���T2H2O��g����H=-483.6kJ•moL-1
�ۣ�H2O��g���TH2O��l����H=-44.0kJ•moL-1
���ݸ�˹���ɣ�����-�ڣ���$\frac{1}{2}$�ɵã�CO��g��+H2O��g��=CO2��g��+H2��g������Ӧ��Ҳ������Ӧ���㣻
��2����ͼ��t3ʱ��˲�������淴Ӧ���ʶ��������淴Ӧ��������϶࣬ƽ�������ƶ�������ӦΪ���������С�ķ��ȷ�Ӧ���ı����������������ѹǿ��Ӧ�������¶ȣ�
t0-t1����ƽ��״̬��t1ʱ˲�������淴Ӧ���ʶ�����������Ӧ����϶࣬ƽ�������ƶ���NH3�ĺ�������t3ʱ�ı������ƽ�������ƶ���t4ʱ����ƽ�⣬NH3�ĺ����ּ�С��t5ʱ�ı���������������������ͬ������ƽ�ⲻ�ƶ���NH3�ĺ������䣻
���¶�ΪT��ʱ����1mol N2��2mol H2�����ݻ�Ϊ0.5L���ܱ������У���ַ�Ӧ����N2��ƽ��ת����Ϊ50%�����ĵĵ���Ϊ0.5mol����
N2��g��+3H2��g��?2NH3��g��
��ʼ����mol����1 2 0
�仯����mol����0.5 1.5 0.5
ƽ������mol����0.5 0.5 0.5
�ٸ���ƽ�ⳣ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$���㣻
������ӦΪ���������С�ķ�Ӧ������ѹǿ��ƽ�������ƶ���ƽ����ϵ�а��������������
����ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���ƽ����ϵ�а������������С��
��3�������°�����HCl����������ˮ���ֽ���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������ϣ�����ǡ����ȫ��Ӧ��������ҺΪNH4Cl��Һ�У���Һ��NH4+����ˮ�⣬��Һ�����ԣ�
��� �⣺��1����֪���٣�2CO��g��+O2��g���T2CO2��g����H=-566.0kJ•moL-1
��.2H2��g��+O2��g���T2H2O��g����H=-483.6kJ•moL-1
�ۣ�H2O��g���TH2O��l����H=-44.0kJ•moL-1
���ݸ�˹���ɣ�����-�ڣ���$\frac{1}{2}$�ɵã�CO��g��+H2O��g��=CO2��g��+H2��g����H=-41.2kJ•mol-1��
�ʴ�Ϊ��CO��g��+H2O��g��=CO2��g��+H2��g����H=-41.2kJ•mol-1��
��2����ͼ��t3ʱ��˲�������淴Ӧ���ʶ��������淴Ӧ��������϶࣬ƽ�������ƶ�������ӦΪ���������С�ķ��ȷ�Ӧ���ı����������������ѹǿ��Ӧ�������¶ȣ�
t0-t1����ƽ��״̬��t1ʱ˲�������淴Ӧ���ʶ�����������Ӧ����϶࣬ƽ�������ƶ���NH3�ĺ�������t3ʱ�ı������ƽ�������ƶ���t4ʱ����ƽ�⣬NH3�ĺ����ּ�С��t5ʱ�ı���������������������ͬ������ƽ�ⲻ�ƶ���NH3�ĺ������䣬ƽ��������NH3�ĺ�����ߵ�һ��ʱ���ǣ�t2-t3ʱ�Σ�
�ʴ�Ϊ�������¶ȣ�t2-t3ʱ�Σ�
���¶�ΪT��ʱ����1mol N2��2mol H2�����ݻ�Ϊ0.5L���ܱ������У���ַ�Ӧ����N2��ƽ��ת����Ϊ50%�����ĵĵ���Ϊ0.5mol����
N2��g��+3H2��g��?2NH3��g��
��ʼ����mol����1 2 0
�仯����mol����0.5 1.5 0.5
ƽ������mol����0.5 0.5 0.5
ƽ�ⳣ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$=$\frac{��\frac{0.5}{2}��^{2}}{\frac{0.5}{2}����\frac{0.5}{2}��^{3}}$=16��
�ʴ�Ϊ��16��
������ӦΪ���������С�ķ�Ӧ������ѹǿ��ƽ�������ƶ���ƽ����ϵ�а��������������ͼ������c���ϣ�
����ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���ƽ����ϵ�а������������С��ͼ������a���ϣ�
�ʴ�Ϊ��c��d��
��3�������°�����HCl����������ˮ���ֽ���ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������ϣ�����ǡ����ȫ��Ӧ��������ҺΪNH4Cl��Һ�У���Һ��NH4+����ˮ�⣬��Һ�����ԣ���Һ������Ũ�ȴ�СΪ��c��Cl-����c��NH4+����c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c��NH4+����c��H+����c��OH-����
���� ���⿼�黯ѧƽ�������Ӱ�����ء��Ȼ�ѧ����ʽ��д������Ũ�ȴ�С�Ƚϵȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д� ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ��
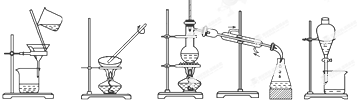
ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ����1��ϡ����Ӧ����1�У���д������ţ���
��2����ʵ��ͨ������A��B��C�������أ��������еĿ����ž����ٹرտ���B������AC�Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п��������ɷ�ֹ���ɵ�������������������
��3����ʵ��ʹ�����ۣ�����Ӧ���ʿ���̫���⣬�����ܻ���ɵIJ�����������۽��뵼�ܴӶ��������ܣ��˿�ɾȥ
��4����FeSO4��Һ�м��루NH4��2SO4������Ʊ���������茶���[��NH4��2SO4•FeSO4•6H2O]��ʽ��Ϊ392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ�ӣ�NH4��2SO4•FeSO4•6H2O�ֲ�Ʒ�����з���������ʵ���D��
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol•L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�ζ��յ�����������һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ��
ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ$\frac{980c}{a}$��100%������ĸac�������ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������BC��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ�
| A�� | ���ˡ�����������Һ | B�� | ���ˡ�������������Һ | ||
| C�� | �����������ˡ���Һ | D�� | ��Һ���������������� |
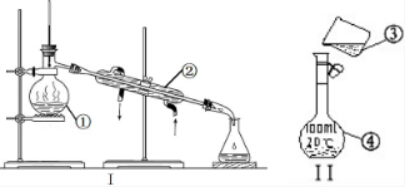 �������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ
�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ