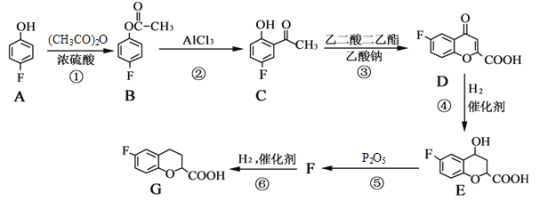
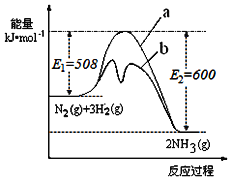
��Ŀ����
����Ŀ��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol/L������ֱ�ΪVa��Vb��HA��Һ��BOH��Һ����ͬ����Ȼ�ϣ�����Va+Vb=100mL��Va��Vb����Һ��pH�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����

A.��c��Ӧ����Һ���У�c(B+)��c(BOH)��0.1molL��1
B.��b��Ӧ����Һ���У�c(B+)��c(A��)
C.���ױ�ʾ����BOH��Һ�������pH�Ĺ�ϵ����
D.���ɵ�a����c�Ĺ��̣�ˮ�ĵ���̶���������С
���𰸡�A
��������
��ͼ����Ϣ��֪��Va=100mLʱ��pH=3��c(H+)=1��10-3mol/L����HAΪ���Vb=100mLʱ��pH=11��c(OH-)=1��10-3mol/L����BOHΪ���������ʼ��pH=3����HA��Һ�м���BOH��Һ��pH���ߣ��������ױ�ʾ����BOH��Һ�������pH�Ĺ�ϵ���ߣ������ҵ���ʼ��pH=11����BOH��Һ�м���HA��Һ��pH���ͣ����������ұ�ʾ����HA��Һ�������pH�Ĺ�ϵ���ߣ��ݴ˷������
A����c��Ӧ����Һ��VamLHA��VbmL��BOH��Һ�Ļ����Һ��Va+Vb=100mL��Vb��Va����50mL��Vb��100mL������n(BA)��0.1mol/L��0.1L=0.01mol����n(B+)+n(BOH)��0.01mol���ʵ�c��Ӧ����Һ��c(B+)+c(BOH)=![]() ��
��![]() =0.1molL-1����A����
=0.1molL-1����A����
B����b��Ӧ����Һ�����ԣ�����ݵ���غ��֪��Һ���У�c(B+)=c(A-)����B��ȷ��
C����HA��Һ�м���BOH��Һ��pH���ߣ��������ױ�ʾ����BOH��Һ�������pH�Ĺ�ϵ���ߣ���C��ȷ��
D����������ˮ�ĵ��룬���ε�ˮ��ٽ�ˮ�ĵ��룬����a��c������ˮ�ĵ���̶���������С����D��ȷ��
��ѡA��