��Ŀ����
����Ŀ���¶�ΪTʱ����2.0 L�����ܱ������г���2.0 mol X����ӦX(g) ![]() Y(g)��Z(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
Y(g)��Z(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
t/s | 0 | 50 | 150 | 250 | 350 |
n(Y)/mol | 0 | 0.32 | 0.38 | 0.40 | 0.40 |
����˵������ȷ����
A. ��Ӧ��ǰ250 s��ƽ������Ϊv(Y)��0.000 8 mol��L��1��s��1
B. ���������������䣬�����¶ȣ�ƽ��ʱc(Y)��0.21 mol��L��1����Ӧ����H>0
C. ��ͬ�¶��£���ʼʱ�������г���4.0 mol Y��4.0 mol Z���ﵽƽ��ʱ����˷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ1/20
D. ��ͬ�¶��£�����ʼʱ�������г���2.0 mol X��0.40 mol Y��0.80 mol Z����ﵽƽ��ǰv(��)>v(��)
���𰸡�D
����������Ӧǰ250 s��ƽ������Ϊv(Y) �� ![]() 0.000 8 mol��L��1��s��1����A��ȷ�����������������䣬�����¶ȣ�ƽ��ʱc(Y)��0.21 mol��L��1��˵��ƽ�������ƶ�����Ӧ����H>0����B��ȷ��ƽ�ⳣ��ֻ���¶��йأ�
0.000 8 mol��L��1��s��1����A��ȷ�����������������䣬�����¶ȣ�ƽ��ʱc(Y)��0.21 mol��L��1��˵��ƽ�������ƶ�����Ӧ����H>0����B��ȷ��ƽ�ⳣ��ֻ���¶��йأ�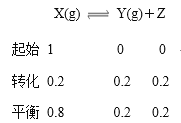
![]() ����C��ȷ����ͬ�¶��£�����ʼʱ�������г���2.0 mol X��0.40 mol Y��0.80 mol Z��
����C��ȷ����ͬ�¶��£�����ʼʱ�������г���2.0 mol X��0.40 mol Y��0.80 mol Z�� ![]() �����Դﵽƽ��ǰv(��)<v(��)����D������
�����Դﵽƽ��ǰv(��)<v(��)����D������

����Ŀ����1����֪��2Fe2O3(s)��3C(s)��3CO2(g)��4Fe(s) ��H1����234.1 kJ��mol��1��
C(s)��O2(g��CO2(g) ��H2����393.5 kJ��mol��1��
��Fe��O2��Ӧ����Fe2O3���Ȼ�ѧ��Ӧ����ʽΪ_______________________________��
(2)��֪H2A��ˮ�д�������ƽ�⣺H2A��H����HA����HA��![]() H����A2����
H����A2����
�ٳ�����NaHA��Һ��pH________(�����)��ԭ����____________________________��
A������7 B��С��7 C������7 D����ȷ��
����֪������H2A�ĸ���(CaA)�ı�����Һ�д�������ƽ�⣺CaA(s)![]() Ca2��(aq)��A2��(aq) ��H>0����Ҫʹ����Һ��Ca2��Ũ�ȱ�С���ɲ�ȡ�Ĵ�ʩ��______________________��
Ca2��(aq)��A2��(aq) ��H>0����Ҫʹ����Һ��Ca2��Ũ�ȱ�С���ɲ�ȡ�Ĵ�ʩ��______________________��
A�������¶� B�������¶� C������NH4Cl���� D������Na2A����
(3)ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ����0.10 mol��L��1 NaOH����Һ���вⶨ�����Ũ�ȵ�ʵ�顣ȡ20.00 mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶ�NaOH����Һ���еζ����ظ������ζ�����2��3�Σ���¼�������¡�
ʵ���� | NaOH��Һ��Ũ��(mol��L��1) | �ζ����ʱ��NaOH��Һ��������(mL) | ������������(mL) |
1 | 0.10 | 22.65 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.93 | 20.00 |
�����������գ�
�ٵζ��ﵽ�յ�ı�־��_____________________________________________��
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ_____________________(������λ��Ч����)��
��������ʵ���У����в���(����������ȷ)����ɲⶨ���ƫ�ߵ���________(����ĸ���)��
A���ζ��յ����ʱ����
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C����ƿˮϴ��δ����
D������NaOH�������Na2CO3����
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
����Ŀ����NA����٤��������ֵ����֪��Ӧ
��1��CH4(g)+2O2(g)�TCO2(g)+2H2O(l) ��H1="a" kJ/mol
��2��CH4(g)+2O2(g)�TCO2(g)+2H2O(g) ��H2="b" kJ/mol���������������
��ѧ�� | C�TO | O�TO | C-H | O-H |
����kJ��mol-1 | 798 | x | 413 | 463 |
����˵����ȷ����
A. �ϱ��� x=(1796+b)/2
B. H2O(g)�TH2O(l) ��S��0����H�T(a-b )kJ/mol
C. ����4NA��C-H������ʱ���÷�Ӧ�ų�����һ��Ϊa kJ
D. ���÷�Ӧ��1����Ƶ�ԭ��ص�⾫��ͭʱ�����������0.2NA������ʱ�����۵���������һ������6.4g

